
a)
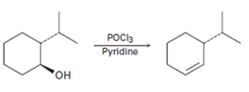
Interpretation:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed. The regiochemistry of the elimination is also to be explained.
Concept introduction:
The dehydration of alcohols to
To propose:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3 is to be proposed.
To explain:
The regiochemistry of the elimination reaction.
b)
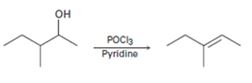
Interpretation:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed. The regiochemistry of the elimination is also to be explained.
Concept introduction:
The dehydration of alcohols to alkenes in the presence of POCl3 follows E2 mechanism. The –OH group is converted into a better leaving group dichlorophosphate, –OPOCl2, when treated with POCl3. Pyridine used as solvent is also a base and it removes a proton arranged anti to the leaving group from the β carbon (requirement for E2 mechanism). The removal of the proton and –OPOCl2 occurs simultaneously in a single step to yield the alkene as the product.
To propose:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed.
To explain:
The regiochemistry of the elimination reaction.
c)
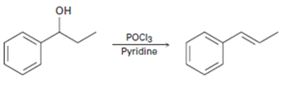
Interpretation:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed. The regiochemistry of the elimination is also to be explained.
Concept introduction:
The dehydration of alcohols to alkenes in the presence of POCl3 follows E2 mechanism. The –OH group is converted into a better leaving group dichlorophosphate, –OPOCl2, when treated with POCl3. Pyridine used as solvent is also a base and it removes a proton arranged anti to the leaving group from the β carbon (requirement for E2 mechanism). The removal of the proton and –OPOCl2 occurs simultaneously in a single step to yield the alkene as the product.
To propose:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed.
To explain:
The regiochemistry of the elimination reaction.
Trending nowThis is a popular solution!

Chapter 17 Solutions
Organic Chemistry
- Draw all of the substitution and elimination products formed from thegiven alkyl halide with each reagent: (a) CH3OH; (b) KOH. Indicate thestereochemistry around the stereogenic centers present in the products,as well as the mechanism by which each product is formed.arrow_forwardDraw the product and indicate the stereochemistry when the given alcohol is treated with each reagent: (a) HBr; (b) PBr3; (c) HCI; (d) SOCI, and pyridine.arrow_forwardBy taking into account electronegativity differences, draw the products formed by heterolysis of the carbon–heteroatom bond in each molecule. Classify the organic reactive intermediate as a carbocation or a carbanion.arrow_forward
- Alkenes can be hydrated to form alcohols by (1) hydroboration followed by oxidation with alkaline hydrogen peroxide and (2) acid-catalyzed hydration. Compare the product formed from each alkene by sequence (1) with those formed from (2). Q.)1-Methylcyclohexenearrow_forwardConsider a reaction where cis-but-2-ene is treated with OsO4 followed by NaHSO3/H2O. Draw the structure of one product that is formed in the reaction, including correct stereochemistry.arrow_forward6) 25pts. Draw the structure of the major alkene product (or products) formed by treatment of each of the following haloalkanes with sodium ethoxide in ethanol. Assume the mechanism is E2 elimination. t-BuO K t-BUOH Br CH3 Eto Na F ETOH CH2CH3 CI H- Eto Na -CH2CH3 ELOH H- ČH3 Br Eto Na ELOH CH3 CI, H Eto Na CH2CH3 H3C H D ELOHarrow_forward
- A 2-bromobutane react with methanol and form a enantiomeric pair of 2-methoxybutane. Draw the structures of the enntiomeric pairs of ethers.arrow_forwardAcid-catalyzed hydration of 2-Methyl-1-butene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explain why, by showing the structures of theproducts.arrow_forwardDraw the organic product obtained by hydroboration-oxidation of each of the following alkenes: (a) trans-2-pentene, (b) 2-tert-butyl-3,3-dimethyl-1- butene, and (c) 1-methylcyclohexene. Having done this, draw the product of the acid-catalyzed hydration of these same alkenes. How do the reaction products differ?arrow_forward
- Explain the different products of the following two reactions by considering the mechanism by which each reaction proceeds. As part of your explanation, use the curved arrow formalism to draw a mechanism for each reaction. CH,OH CH2=CH-CH-CH, + Na*¯OCH, CH;=CH-CH-CH3 Br OCH, CH,=CH-CH-CH, + CH,OH – CH,=CH–CH–CH, + CH,CH=CHCH, Br OCH, OCH,arrow_forward1) 3- Methyl-2-butanol will react with H2SO4 to give two isomeric alkenes. Write suitable reaction mechanism to show how these isomeric products are achieved and indicate the major product. Write the IUPAC name for each product.arrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forward
