
Chemistry (7th Edition)
7th Edition
ISBN: 9780321943170
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.147CP
The acidity of lemon juice is derived primarily from citric acid (
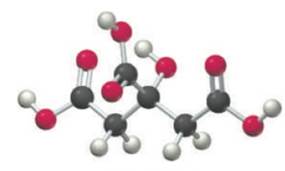
Citric acid
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Chemistry (7th Edition)
Ch. 16 - Prob. 16.1PCh. 16 - APPLY 16.2 Write balanced net ionic equations for...Ch. 16 - PRACTICE 16.3 Calculate the concentrations of all...Ch. 16 - APPLY 16.4 Calculate the pH of a solution prepared...Ch. 16 - Conceptual PRACTICE 16.5 The following pictures...Ch. 16 - Conceptual APPLY 16.6 The following pictures...Ch. 16 - Prob. 16.7PCh. 16 - Prob. 16.8ACh. 16 - Prob. 16.9PCh. 16 - PRACTICE 16.10 Use the Henderson-Hasselbalch...
Ch. 16 - APPLY 16.11 The of the amine group of the amino...Ch. 16 - PRACTICE 16.12 How would you prepare anbuffer...Ch. 16 - APPLY 16.13 Suppose you are performing an...Ch. 16 - Prob. 16.14PCh. 16 - APPLY 16.15 A 40.0 mL volume of 0.100 M NaOH is...Ch. 16 - Prob. 16.16PCh. 16 - Prob. 16.17ACh. 16 - Prob. 16.18PCh. 16 - Prob. 16.19ACh. 16 - PRACTICE 16.20 Write the equilibrium-constant...Ch. 16 - Prob. 16.21ACh. 16 - Prob. 16.22PCh. 16 - Prob. 16.23ACh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25ACh. 16 - Prob. 16.26PCh. 16 - Prob. 16.27ACh. 16 - Prob. 16.28PCh. 16 - Prob. 16.29PCh. 16 - Prob. 16.30ACh. 16 - Prob. 16.31PCh. 16 - Prob. 16.32ACh. 16 - Prob. 16.33PCh. 16 - Prob. 16.34ACh. 16 - PROBLEM 16.35 Determine whether Cd2+ can be...Ch. 16 - Prob. 16.36PCh. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40CPCh. 16 - The following pictures represent initial...Ch. 16 - Prob. 16.42CPCh. 16 - The following pictures represent solutions at...Ch. 16 - The following pictures represent solutions at...Ch. 16 - Prob. 16.45CPCh. 16 - Prob. 16.46CPCh. 16 - Prob. 16.47CPCh. 16 - Prob. 16.48CPCh. 16 - Prob. 16.49CPCh. 16 - 16.50 Is the pH greater than, equal to, or less...Ch. 16 - Is the pH greater than, equal to, or less than 7...Ch. 16 - Prob. 16.52SPCh. 16 - Prob. 16.53SPCh. 16 - Prob. 16.54SPCh. 16 - Prob. 16.55SPCh. 16 - 16.56 The equilibrium constant for the...Ch. 16 - 16.57 The equilibrium constant for the...Ch. 16 - 16.58 Does the pH increase, decrease, or remain...Ch. 16 - 16.59 Does the pH increase, decrease, or remain...Ch. 16 - 16.60 Calculate the pH of a solution that is 0.25...Ch. 16 - Prob. 16.61SPCh. 16 - Prob. 16.62SPCh. 16 - The pH of a solution of NH3 and NH4Br is 8.90....Ch. 16 - Prob. 16.64SPCh. 16 - Prob. 16.65SPCh. 16 - Prob. 16.66SPCh. 16 - Which of the following gives a buffer solution...Ch. 16 - Prob. 16.68SPCh. 16 - Prob. 16.69SPCh. 16 - Prob. 16.70SPCh. 16 - Prob. 16.71SPCh. 16 - Prob. 16.72SPCh. 16 - Calculate the pH of 0.375 L of a 0.18 M acetic...Ch. 16 - Prob. 16.74SPCh. 16 - Prob. 16.75SPCh. 16 - Prob. 16.76SPCh. 16 - Prob. 16.77SPCh. 16 - Prob. 16.78SPCh. 16 - Prob. 16.79SPCh. 16 - Prob. 16.80SPCh. 16 - Prob. 16.81SPCh. 16 - Prob. 16.82SPCh. 16 - Prob. 16.83SPCh. 16 - Prob. 16.84SPCh. 16 - Prob. 16.85SPCh. 16 - Prob. 16.86SPCh. 16 - Prob. 16.87SPCh. 16 - Prob. 16.88SPCh. 16 - Prob. 16.89SPCh. 16 - Prob. 16.90SPCh. 16 - Prob. 16.91SPCh. 16 - Prob. 16.92SPCh. 16 - Prob. 16.93SPCh. 16 - Prob. 16.94SPCh. 16 - Prob. 16.95SPCh. 16 - Prob. 16.96SPCh. 16 - 16.97 What is the pH at the equivalence point for...Ch. 16 - Prob. 16.98SPCh. 16 - Prob. 16.99SPCh. 16 - Prob. 16.100SPCh. 16 - Prob. 16.101SPCh. 16 - Prob. 16.102SPCh. 16 - Prob. 16.103SPCh. 16 - Prob. 16.104SPCh. 16 - Prob. 16.105SPCh. 16 - Prob. 16.106SPCh. 16 - Prob. 16.107SPCh. 16 - Use Le Châtelier’s principle to explain the...Ch. 16 - Use Le Châtelier’s principle to predict whether...Ch. 16 - Calculate the molar solubility of PbCrO4 in: (a)...Ch. 16 - Prob. 16.111SPCh. 16 - Prob. 16.112SPCh. 16 - Prob. 16.113SPCh. 16 - Prob. 16.114SPCh. 16 - Prob. 16.115SPCh. 16 - Prob. 16.116SPCh. 16 - Dissolution of 5.010-3 mol of CrOH3 in 1.0L of...Ch. 16 - Prob. 16.118SPCh. 16 - Prob. 16.119SPCh. 16 - Prob. 16.120SPCh. 16 - Prob. 16.121SPCh. 16 - Prob. 16.122SPCh. 16 - Prob. 16.123SPCh. 16 - Prob. 16.124SPCh. 16 - Prob. 16.125SPCh. 16 - Prob. 16.126SPCh. 16 - Prob. 16.127SPCh. 16 - Prob. 16.128SPCh. 16 - Prob. 16.129SPCh. 16 - Prob. 16.130SPCh. 16 - Prob. 16.131SPCh. 16 - Prob. 16.132CPCh. 16 - Prob. 16.133CPCh. 16 - Prob. 16.134CPCh. 16 - Prob. 16.135CPCh. 16 - Prob. 16.136CPCh. 16 - Prob. 16.137CPCh. 16 - Prob. 16.138CPCh. 16 - Prob. 16.139CPCh. 16 - Prob. 16.140CPCh. 16 - Prob. 16.141CPCh. 16 - Prob. 16.142CPCh. 16 - Prob. 16.143CPCh. 16 - Prob. 16.144CPCh. 16 - Prob. 16.145CPCh. 16 - Prob. 16.146CPCh. 16 - The acidity of lemon juice is derived primarily...Ch. 16 - Prob. 16.148CPCh. 16 - Prob. 16.149CPCh. 16 - Prob. 16.150CPCh. 16 - Prob. 16.151CPCh. 16 - Prob. 16.152MPCh. 16 - Prob. 16.153MPCh. 16 - Prob. 16.154MPCh. 16 - Prob. 16.155MPCh. 16 - Prob. 16.156MPCh. 16 - Prob. 16.157MPCh. 16 - Prob. 16.158MPCh. 16 - In qualitative analysis, Ca2+ and Ba2+ are...Ch. 16 - Prob. 16.160MPCh. 16 - Prob. 16.161MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two strategies are also followed when solving for the pH of a base in water. What is the strategy for calculating the pH of a strong base in water? List the strong bases mentioned in the text that should be committed to memory. Why is calculating the pH of Ca(OH)2 solutions a little more difficult than calculating the pH of NaOH solutions? Most bases are weak bases. The presence of what element most commonly results in basic properties for an organic compound? What is present on this element in compounds that allows it to accept a proton? Table 13-3 and Appendix 5 of the text list Kb values for some weak bases. What strategy is used to solve for the pH of a weak base in water? What assumptions are made when solving for the pH of weak base solutions? If the 5% rule fails, how do you calculate the pH of a weak base in water?arrow_forwardConsider the following ions: NH4+, CO32, Br, S2, and ClO4. (a) Which of these ions in water gives an acidic solution and which gives a basic solution? (b) Which of these anions will have no effect on the pH of an aqueous solution? (c) Which ion is the strong base? (d) Write a chemical equation for the reaction of each basic anion with water.arrow_forwardA chemist wanted to determine the concentration of a solution of lactic acid, HC3H5O3. She found that the pH of the solution was 2.60. What was the concentration of the solution? The Kd of lactic acid is 1.4 104.arrow_forward
- Lactic acid, C3H6O3, occurs in sour milk as a result of the metabolism of certain bacteria. Calculate the pH of a solution of 56. mg lactic acid in 250. mL water. Ka for D-lactic acid is 1.5 × 10−4.arrow_forwardIonization of the first proton from H2SO4 is complete (H2SO4 is a strong acid); the acid-ionization constant for the second proton is 1.1 102. a What would be the approximate hydronium-ion concentration in 0.100 M H2SO4 if ionization of the second proton were ignored? b The ionization of the second proton must be considered for a more exact answer, however. Calculate the hydronium-ion concentration in 0.100 M H2SO4, accounting for the ionization of both protons.arrow_forwardA solution of acetic acid, HC2H3O2, on a laboratory shelf was of undetermined concentration. If the pH of the solution was found to be 2.57, what was the concentration of the acetic acid? The Ka of acetic acid is 1.7 105.arrow_forward
- For conjugate acidbase pairs, how are Ka and Kb related? Consider the reaction of acetic acid in water CH3CO2H(aq)+H2O(l)CH3CO2(aq)+H3O+(aq) where Ka = 1.8 105 a. Which two bases are competing for the proton? b. Which is the stronger base? c. In light of your answer to part b. why do we classify the acetate ion (CH3CO2) as a weak base? Use an appropriate reaction to justify your answer. In general, as base strength increases, conjugate acid strength decreases. Explain why the conjugate acid of the weak base NH3 is a weak acid. To summarize, the conjugate base of a weak acid is a weak base and the conjugate acid of a weak base is a weak acid (weak gives you weak). Assuming Ka for a monoprotic strong acid is 1 106, calculate Kb for the conjugate base of this strong acid. Why do conjugate bases of strong acids have no basic properties in water? List the conjugate bases of the six common strong acids. To tie it all together, some instructors have students think of Li+, K+, Rb+, Cs+, Ca2+, Sr2+, and Ba2+ as the conjugate acids of the strong bases LiOH, KOH. RbOH, CsOH, Ca(OH)2, Sr(OH)2, and Ba(OH)2. Although not technically correct, the conjugate acid strength of these cations is similar to the conjugate base strength of the strong acids. That is, these cations have no acidic properties in water; similarly, the conjugate bases of strong acids have no basic properties (strong gives you worthless). Fill in the blanks with the correct response. The conjugate base of a weak acid is a_____base. The conjugate acid of a weak base is a_____acid. The conjugate base of a strong acid is a_____base. The conjugate acid of a strong base is a_____ acid. (Hint: Weak gives you weak and strong gives you worthless.)arrow_forwardExplain why the pH does not change significantly when a small amount of an acid or a base is added to a solution that contains equal amounts of the acid H3PO4 and a salt of its conjugate base NaH2PO4.arrow_forwardEstimate the pH that results when the following two solutions are mixed. a) 50 mL of 0.3 M CH3COOH and 50 mL of 0.4 M KOH b) 100 mL of 0.3 M CH3COOH and 50 mL of 0.4 M NaOH c) 150 mL of 0.3 M CH3COOH and 100 mL of 0.3 M Ba(OH)2 d) 200 mL of 0.3 M CH3COOH and 100 mL of 0.3 M Ba(OH)2arrow_forward
- Strong Acids, Weak Acids, and pH Two 0.10-mol samples of the hypothetical monoprotic acids HA(aq) and HB(aq) are used to prepare 1.0-L stock solutions of each acid. a Write the chemical reactions for these acids in water. What are the concentrations of the two acid solutions? b One of these acids is a strong acid, and one is weak. What could you measure that would tell you which acid was strong and which was weak? c Say that the HA(aq) solution has a pH of 3.7. Is this the stronger of the two acids? How did you arrive at your answer? d What is the concentration of A(aq) in the HA solution described in part c? e If HB(aq) is a strong acid, what is the hydronium-ion concentration? f In the solution of HB(aq), which of the following would you expect to be in the greatest concentration: H3O+(aq), B(aq), HB(aq), or OH(aq)? How did you decide? g In the solution of HA(aq), which of the following would you expect to be in the greatest concentration: H3O+(aq), A+(aq), HA(aq), or OH(aq)? How did you decide? h Say you add 1.0 L of pure water to a solution of HB. Would this water addition make the solution more acidic, make it less acidic, or not change the acidity of the original solution? Be sure to fully justify your answer. i You prepare a 1.0-L solution of HA. You then take a 200-mL sample of this solution and place it into a separate container. Would this 200 mL sample be more acidic, be less acidic, or have the same acidity as the original 1.0-L solution of HA(aq)? Be sure to support your answer.arrow_forwardAcids You make a solution by dissolving 0.0010 mol of HCl in enough water to make 1.0 L of solution. a Write the chemical equation for the reaction of HCl(aq) and water. b Without performing calculations, give a rough estimate of the pH of the HCl solution. Justify your answer. c Calculate the H3O+ concentration and the pH of the solution. d Is there any concentration of the base OH present in this solution of HCl(aq)? If so, where did it come from? e If you increase the OH concentration of the solution by adding NaOH, does the H3O+ concentration change? If you think it does, explain why this change occurs and whether the H3O+ concentration increases or decreases. f If you were to measure the pH of 10 drops of the original HCl solution, would you expect it to be different from the pH of the entire sample? Explain. g Explain how two different volumes of your original HCl solution can have the same pH yet contain different moles of H3O+. h If 1.0 L of pure water were added to the HCl solution, would this have any impact on the pH? Explain.arrow_forwardMost naturally occurring acids are weak acids. Lactic acid is one example. CH3CH(OH)CO2H(s)+H2O(l)H3O+(aq)+CH3CH(OH)CO2(aq) If you place some lactic acid in water, it will ionize to a small extent, and an equilibrium will be established. Suggest some experiments to prow that this is a weak acid and that the establishment of equilibrium is a reversible process.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY