
Concept explainers
Interpretation:
The benzophenone n-Π* triplet T1 without having benzophenone pass through its first singlet stateneeds to be determined.
Concept Introduction:
In the singlet state; the orientation of electrons has the same spin while in the triplet state; the orientation of electrons has the opposite spin.
Explanation of Solution
The reaction between benzophenone and isopropyl alcohol is shown below:
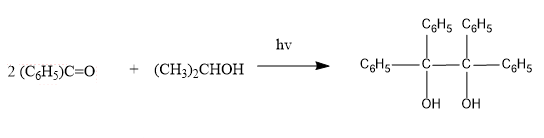
The quantum yield for the given reaction is a unity which states that each molecule of excited benzophenone gives one molecule of benzo pinacol. In the given reaction; fluorescence is not observed, a triplet excited state is involved. n-Π* triplet excited state takes a hydrogen atom from isopropanol.
The mechanism of the given reaction is shown below:
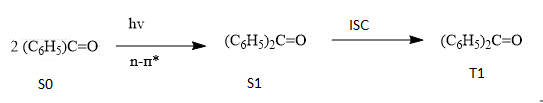
Where,
S0 = singlet ground state
S1 = first singlet excited state
T1 = first triplet excited state
ISC = intersystem crossing
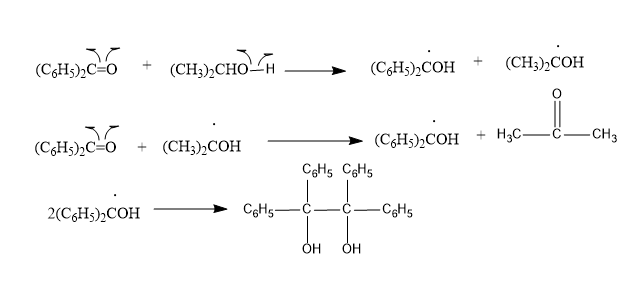
According to the mechanism; it shows that the singlet excited state of benzophenone gores through intersystem crossing to triplet state very readily. Therefore, it is the triplet state that is involved.
Want to see more full solutions like this?
Chapter 54 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- The following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forwardWhen pent-1-ene is treated with mercury(II) acetate in methanol and the resulting product is reacted with NaBH4, what is the primary organic compound which results? 1-ethoxypentane 1-methoxypentane 3-ethoxypentane 2-ethoxypentane 2-methoxypentanearrow_forwardTreatment of trans-2-chlorocyclohexanol with NaOH yields 1, 2-epoxy-cyclohexane, but reaction of the cis isomer under the same conditions yields cyclohexanone. Propose mechanisms for both reactions, and explain why the different results are obtained.arrow_forward
- Which of the following starting materials will provide the desired compound under the proposed conditions: م O کنند امام احمد محمد ہ میر O ه A B U E starting material A ATP, H2O OH OH B م کی نو عليك حد E OHarrow_forwardN-Nitrosamines by themselves are not significant carcinogens. However, they are activated in the liver by a class of iron-containing enzymes (members of the cytochrome P-450 family). Activation involves the oxidation of a C-H bond next to the amine nitrogen to a C-OH group. OH ОН N=0 N=O Og 'N- H+ H N,+ cyt P-450 N-Nitroso- 2-Нydroxy-N- nitrosopiperidine An alkyl diazonium ion piperidine (a carcinogen) Show how this hydroxylation product can be transformed into an alkyl diazonium ion, an active alkylating agent and therefore a carcinogen, in the presence of an acid catalyst.arrow_forwardShow how you might prepare the anti- inflammatory agent ibuprofen starting from isobutyl benzene. More than one step is needed. Isobutylbenzene Ibuprofen CO₂Harrow_forward
- Reaction of HBr with 2-methylpropene yields 2-bromopropane. What is the structure of the carbocation intermediate formed during the reaction? Show the mechanism of the reaction.arrow_forwardN Ph :0: CH3 Primary amines add to aldehydes and ketones to give imines. Imines are formed in a reversible, acid-catalyzed process that begins with nucleophilic addition of the primary amine to the carbonyl group, followed by transfer of the proton to yield a neutral carbinolamine. Protonation of the hydroxyl group converts it into a good leaving group and an E1-like loss of water yields an iminium ion. Deprotonation yields the product imine and regenerates the acid catalyst. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions CH3 NH₂OH NH₂OH Ph- :0: OH CH3 CH3 36arrow_forwardThe following nucleophilic substitution reaction has been reported in the chemical literature. Using what you know about nucleophilic substitution in simple systems, predict the major product of the reaction. NE Cl H₂O HO Click and drag to start drawing a structure. 0 D 0arrow_forward
- Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardNonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardNitriles, R–=C≡N, undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the compound produced by hydrolysis of propanenitrile, CH3CH2C≡N, if it has IR absorptions from 2500–3100 cm-1 and at 1710 cm-1, and has M+=74?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

