
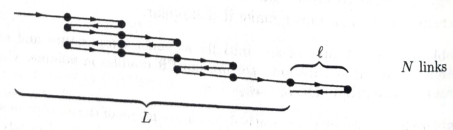
(a) Find an expression for the entropy of this system in term of N and
(b) Write down a formula for L in terms of N and N1,
(c) For a one-dimensional system such as this, the length L is analogous to the volume V of a three-dimensional system. Similarly, the pressure P is replaced by the tension force F. Taking F to be positive when the rubber band is pulling inward, write down and explain the appropriate
(d) Using the thermodynamic Identity, you can flow express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T, N, and
(e) Show that when
(f) Discuss the dependence of the tension force on temperature. If you increase the temperature of a rubber band, does it tend to expand or contract? Does this behavior make sense?
(g) Suppose that you hold a relaxed rubber band in both hands and suddenly stretch it. Would you expect its temperature to increase or decrease? Explain. Test your prediction with a real rubber band (preferably a fairly heavy one with lots of stretch), using your lips or forehead as a thermometer. (Hint: The entropy you computed in part (a) is not the total entropy of the rubber band. There is additional entropy a8sociated with the vibrational energy of the molecules; this entropy depends on U but is approximately independent of L.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
An Introduction to Thermal Physics
Additional Science Textbook Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Life in the Universe (4th Edition)
Conceptual Integrated Science
Physics: Principles with Applications
Conceptual Physics (12th Edition)
Applied Physics (11th Edition)
- The simple mass on a spring is the basis for our description of almost any oscillation. Lets consider the vibrational states of a simple diatomic molecule, say N2. We will model it as two identical masses connected by a spring as shown in the figure at the top right.This is a little different from the cart on a spring since there is no wall -- both of the atoms are moving. But if we only look at oscillations of the molecule where the center of mass is not moving, the atoms are moving equally and oppositely. When one is going left, the other goes an equal amount to the right and vice versa. This is like two carts attached to a wall and oscillating equally and oppositely as shown in the lower picture. The only difference is the way we treat the spring. When each atom moves a distance x, the spring between the atoms actually stretches a distance 2x, so the force on each atom is -2kx. We can therefore model the motion of one of the nitrogen atoms as a single cart on a spring if we…arrow_forwardI'm hoping for a good explanation of how to do this. I'm also wondering why it matters if the configuration is linear or nonlinear? A triatomic molecule can have a linear configuration, as does CO2 (Figure a), or it can be nonlinear, like H2O (Figure b). Suppose the temperature of a gas of triatomic molecules is sufficiently low that vibrational motion is negligible. (a) What is the molar specific heat at constant volume, expressed as a multiple of the universal gas constant (R) if the molecules are linear?Eint/nT = ? (b) What is the molar specific heat at constant volume, expressed as a multiple of the universal gas constant (R) if the molecules are nonlinear?Eint/nT = ? At high temperatures, a triatomic molecule has two modes of vibration, and each contributes 0.5R to the molar specific heat for its kinetic energy and another 0.5R for its potential energy. (c) Identify the high-temperature molar specific heat at constant volume for a triatomic ideal gas of the linear molecules. (Use…arrow_forwardConsider two blocks of copper. Block A contains 800 atoms and initially has a total of 20 quanta of energy. Block B contains 200 atoms and initially has 80 quanta of energy. The two blocks are placed in contact with each other, inside an insulated container (so no thermal energy can be exchanged with the surroundings).After waiting for a long time (for example, an hour), which of the following would you expect to be true? A. The entropy of block A is equal to the entropy of block B. B. Approximately 50 quanta of energy are in block A, and approximately 50 quanta of energy are in block B. C. Approximately 80 quanta of energy are in block A, and approximately 20 quanta of energy are in block B. D. The temperature of block A and the temperature of block B are equal.arrow_forward
- Consider Ω = αU^(αNV) Use the following steps to find T, U(T), and CV 1. Use quantum mechanics and some combinatorics to find an expression for the multiplicity,Ω , in terms of U, V, N, and any other relevant variables. 2. Take the logarithm to find the entropy, S. 3. Differentiate S with respect to U and take the reciprocal to find the temperature, T, as a function of U and other variables. 4. Solve for U as a function of T (and other variables). 5. Differentiate U(T) to obtain a prediction for the heat capacity (with the other variables held fixed).arrow_forwardThree balls (labeled A, B, C) are placed into two different boxes (1 and 2), as in (Figure 1). If all arrangements are equally likely, what is the probability that all three will be in box 1?arrow_forwardWhy is the probability of a system's ground state larger than the probabilities of its excited states for a system in thermal equilibrium with a reservoir? In answering this you may want to consider your calculations in this worksheet. a. Adding energy to the large reservoir increases the total entropy more than adding the same amount of energy to the system. b. Removing all energy from the system increases its entropy the most. c. The probability of the state of the system is maximized when the number of microstates of the system is maximized. d. The probability of the state of the system is maximized when the number of microstates for that state of the system and reservoir is maximized.arrow_forward
- A gas consists of three atoms with access to three different quantum states with the same energy. How many different microstates can be formed from these quantum levels for the case of the Fermi-Dirac gas, where the atoms are differentiated but only one atom is allowed in each state.arrow_forwardThe intensities of spectroscopic transitions between the vibrational states of a molecule are proportional to the square of the integral ∫ψv′xψvdx over all space. Use the relations between Hermite polynomials given in Table 7E.1 to show that the only permitted transitions are those for which v′ = v ± 1 and evaluate the integral in these cases.arrow_forwardShow me the step by step solution. Thank you!arrow_forward
- A system containing a single particle in a rigid container at temperature 100 K has a translational partition function of Ztrans = 1.5 × 1012. (a) If the mass of the particle is doubled, what is the new value for ztrans? Report your answer in scientific notation (in Blackboard, 1.23 x 1045 is written 1.23E45).arrow_forwardConsider N identical harmonic oscillators (as in the Einstein floor). Permissible Energies of each oscillator (E = n h f (n = 0, 1, 2 ...)) 0, hf, 2hf and so on. A) Calculating the selection function of a single harmonic oscillator. What is the division of N oscillators? B) Obtain the average energy of N oscillators at temperature T from the partition function. C) Calculate this capacity and T-> 0 and At T-> infinity limits, what will the heat capacity be? Are these results consistent with the experiment? Why? What is the correct theory about this? D) Find the Helmholtz free energy from this system. E) Derive the expression that gives the entropy of this system for the temperature.arrow_forwardFor an ideal gas of classical non- interacting atoms in thermal equilibrium, the Cartesian component of the velocity are statistically independent. In three dimensions, the probability density distribution of the velocity is: where σ² = kBT m P(Vx, Vy, Vz) = (2nо²)-³/² exp 20² 1. Show that the probability density of the velocity is normalized. 2. Find an expression of the arithmetic average of the speed. 3. Find and expression of the root-mean-square value of the speed. 4. Estimate the standard deviation of the speed.arrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
