
(a)
Interpretation:
The pH at which the zwitter ion [Z] and the cationic form [C+] of the amino acid alanine become equal needs to be calculated.
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main
functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value. - Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 2.29
Explanation of Solution
The given amino acid is alanine which has the following structure:
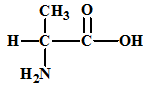
The structures of the respective cationic form (C+), zwitter ion (Z) and the anionic form (A-) are:
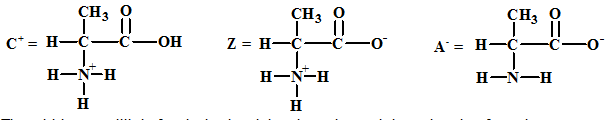
The acid-base equilibria for alanine involving the cation and the zwitter ion forms is:
(b)
Interpretation:
The pH at which the zwitter ion [Z] and the anionic form [A-] of the amino acid alanine become equal needs to be calculated
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value.
- Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 9.74
Explanation of Solution
The given amino acid is alanine which has the following structure:
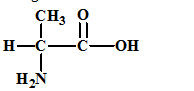
The structures of the respective cationic form (C+), zwitter ion (Z) and the anionic form (A-) are:
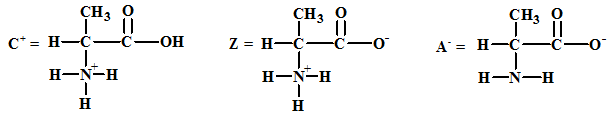
The acid-base equilibria for alanine involving the anion and the zwitter ion forms is:
(c)
Interpretation:
The pH at the isoelectric point needs to be calculated
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value.
- Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 6.02
Explanation of Solution
For alanine at the isoelectric point:
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Principles and Reactions
- (b) Calculate the pH of 0.0005 mol dm-3 ethanoic acid when its pKa = 4.75 and explain the assumptions made in the calculations CH3COOH(aq) CH3COO-(aq) + H+(aq) Ka = pH =arrow_forwardIf Phenolphthalein was added to H2O (l) + CO2 (g) → H2CO3 (aq) what color would the solution be?arrow_forwardThere are many organic acids and bases in our cells, and their presence modifies the pH of the fluids inside them. It is useful to be able to assess the pH of solutions of acids and bases and to make inferences from measured values of the pH. A solution of equal concentrations of lactic acid and sodium lactate was found to have pH= 3.08. (a) What are the values of pKa and Ka of lactic acid? (b) What would the pH be if the acid had twice the concentration of the salt?arrow_forward
- A weak acid, HA, has a pKa = 6.2. If a solution of NaA(aq) is prepared, what does the pH of the solution need to be adjusted to in order to achieve the following ratios of deprotonated to protonated species? 1) [A-] / [HA] = 0.01 2) [A-] / [HA] = 10 3) [A-] / [HA] = 1 4) [A-] / [HA] = 106 Note: NaA is the sodium salt of , the conjugate base of HA. Assume NaA completely dissociates in water.arrow_forwardMg(OH)2(s) Mg2+(aq) + 2OH- (aq) Write the balanced net ionic equation for the equilbriumarrow_forward(a) Give the conjugate base of the following Brønsted–Lowry acids: (i) HIO3, (ii) NH4+. (b) Give the conjugate acidof the following Brønsted–Lowry bases: (i) O2-, (ii) H2PO4-.arrow_forward
- Good explanation Asap Thanks Hemoglobin, Hb, has four Fe atoms per α molecule that, on average, bind about three O2 molecules. Hb(aq)+3O 2 (g) = Hb(O 2 ) 3 (aq)Discuss mountain or space sickness (high altitude) in terms of this balance.arrow_forwardPredict the products from the following chemical reactions. (a) HNO3 + Ba(OH)2 ⟶ (b) CaCl2 + CsOH ⟶ (c) Ammonium Phosphate + Magnesium Sulfate ⟶arrow_forwardA monoprotic weak acid,HA, dissociates in water according to the reaction HA(aq)+H2O equilibrium arrows H3O+(aq)+A-(aq) The equilibrium concentrations of the reactants and products are [HA]=0.230M, [H3O+]=4.00•10^-4 and [A-]=4.00•10^-4. Calculate the Ka value for the acid HA.arrow_forward
- 5) Find the concentration of H30*(aq) in a 1.75 M solution of lactic acid, HC3H5O3, at 25°C. Ka= 1.38 x 10*. 6) Write the equilibrium expression for the ionization of HOI, and calculate the concentration of HOI(aq) in solution if [H3O*]=2.3 x 10° M and pKa = 10.7 at 25°C.arrow_forward(f) Calculate the pH of the following solutions: (i) A 0.045 M solution of sulphuric acid. (ii) A solution containing 0.4 g sodium hydroxide in 100 cm³ water. (iii) Given the Ka value for CH3COOH is 1.8 x 10-5, calculate the pH of the acid. The initial concentration of the acid is 0.2 Marrow_forwardWrite the expression Kc for the following reactions. (a) Fe2+(aq) + Ce4+(aq) ⇋ Fe3+(aq) + Ce3+(aq) (b) CaCO3(s) ⇋ CaO(s) + CO3(g)arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

