
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.1B, Problem 2.3P
For each of the following compounds
- 1. Draw the Lewis structure.
- 2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment.
- 3. Estimate whether the compound will have a large small, or zero dipole moment.
- a. NH4+
- b. O3
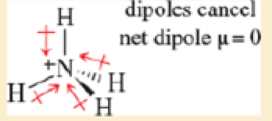
- a. NH4+ has four polar N—H bonds These bonds are probably more polarized than a typical N—H bond, because the N in NH4+ bears a formal positive charge Nevertheless, these four colar bonds have a symmetric tetrahedral arrangement so they cancel each other.
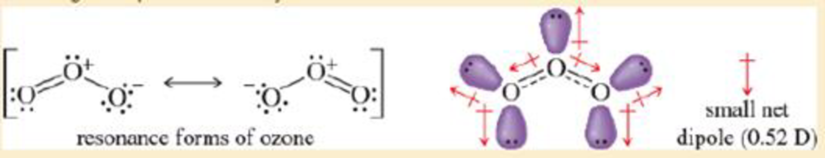
- b. Ozone (O3) is an sp2 hybrid structure with a lone pair on the central oxygen atom Therefore O3 must be bent. The resonance structures imply partia negatve charges on the outer oxygens and a partial positive charge on the central oxygen. The lone pair on the central oxygen cancels part, but not all, of the vector sum of the two O—O dipoles The resulting not d polo is relatively small.
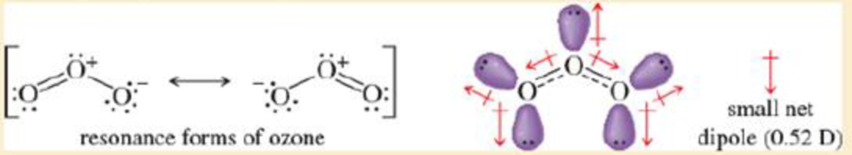
- a. Ozone (O3) is an sp2 hybrid structure, with a lone pair on the central oxygon atom Therefore, O3 must be bent. The resonance structures imply partial negative charges on the outer oxygens end a partial positive charge on the central oxygen. The lone pair on the central oxygen cancels part but not all, of the vector sum of the two O—O dipoles The resulting net dipole is relatively small.
- b. CH2CI2
- c. CH3F
- d. CF4
- e. CH3OH
- f. HCN
- g. CH3CHO
- h. H2C=NH
- i. (CH3)3N
- j. CH2=CHCI
- k. BF3
- l. BeCl2
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sodium Azide is an ionic compound that is used in automotive air bags. It has the chemical
formula NaN3 (The Azide ion is N , and should not be confused with the Nitride ion,
N°).
a. Give the LEWIS diagram of the Azide ion.
b. What is the geometry of the Azide ion? What is the bond angle of the Azide
ion?
The formula for nitryl chloride is CINO2 (in which N is the central atom).
a.Draw the Lewis structure for the molecule, including all resonance structures.
b.What is the N-O bond order?
c.Describe the electron-pair and molecular geometries and give values for all bond angles.
d.What is the most polar bond in the molecule? Is the molecule polar?
e.The computer program used to calculate electrostatic potential surfaces gave the following charges on atoms in the molecule: A =-0.03, B = -0.26, and C = +0.56. Identify the atoms A, B, and C. Are these calculated charges in accord with your predictions?
12. The presence of a lone pair in the structure of ammonia (NH ) causes the H-N-H bond angle to deviate from the ideal value of 109.5° for tetrahedral geometry. What is the expected deviation for the value of the H-N-H bond angle?
a. H-N-H bond angle > 109.5°b. it will not deviate from the ideal valuec. H-N-H bond angle < 109.5°d. cannot be determined
13. Which of the following statements are TRUE regarding the valence shell electron pair repulsion (VSEPR) theory? (Choose all that applies.
a. 3D arrangement of the nuclei joined by electron groups result to the molecular shapeb. each electron group repels other groups to minimize the angle between themc. electron group arrangements are defined by both the bonding and nonbonding valence electron groups around the central atom
14. TRUE/FALSE: Secondary bonds are stronger than primary bonds due to the longer distance between the molecules involved in the interactions.
Chapter 2 Solutions
Organic Chemistry (9th Edition)
Ch. 2.1A - Prob. 2.1PCh. 2.1B - The NF bond is more polar than the NH bond: but...Ch. 2.1B - For each of the following compounds 1. Draw the...Ch. 2.1B - Two isomers of 1,2-dichloroethene are known One...Ch. 2.2C - Prob. 2.5PCh. 2.2C - Prob. 2.6PCh. 2.3 - Prob. 2.7PCh. 2.4 - Calculate the pH of the following solutions a....Ch. 2.6A - Ammonia appears in Table 2-2 as both an acid and a...Ch. 2.7 - Write equations for the following acid-base...
Ch. 2.7 - Ethanol, methylamine. and acetic acid are all...Ch. 2.8 - Prob. 2.12PCh. 2.10 - Write equations for the following acid-base...Ch. 2.10 - Rank the following acids in decreasing order of...Ch. 2.11 - Prob. 2.15PCh. 2.11 - Prob. 2.16PCh. 2.11 - Consider each pair of bases and explain which one...Ch. 2.12 - Which is a stronger base ethoxide ion or acetate...Ch. 2.12 - Prob. 2.19PCh. 2.12 - Prob. 2.20PCh. 2.12 - Prob. 2.21PCh. 2.12 - Choose the more basic member of each pair of...Ch. 2.14 - Prob. 2.23PCh. 2.15D - Classify the following hydrocarbons and draw a...Ch. 2.16D - Prob. 2.25PCh. 2.17C - Draw a Lewis structure and classify each of the...Ch. 2.17C - Circle the functional groups in the following...Ch. 2 - The CN triple bond in acetonitrile has a dipole...Ch. 2 - Prob. 2.29SPCh. 2 - Sulfur dioxide has a dipole moment of 1.60 D....Ch. 2 - Which of the following pure compounds can form...Ch. 2 - Predict which member of each pair is more soluble...Ch. 2 - Prob. 2.33SPCh. 2 - Prob. 2.34SPCh. 2 - Predict which compound in each pair has the higher...Ch. 2 - All of the following compounds can react as acids...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - The Ka of phenylacetic acid is 5 2 105, and the...Ch. 2 - The following compound can become protonated on...Ch. 2 - The following compounds are listed in increasing...Ch. 2 - Prob. 2.42SPCh. 2 - Prob. 2.43SPCh. 2 - Compare the relative acidity of 1-molar aqueous...Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - The following compounds can all react as bases. a....Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - Prob. 2.48SPCh. 2 - Methyllithium (CH3Li) is often used as a base in...Ch. 2 - Label the reactants in these acid-base reactions...Ch. 2 - In each reaction, label the reactants as Lewis...Ch. 2 - Prob. 2.52SPCh. 2 - Each of these compounds can react as a nucleophile...Ch. 2 - Prob. 2.54SPCh. 2 - Give a definition and an example for each class of...Ch. 2 - Circle the functional groups in the following...Ch. 2 - Prob. 2.57SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of these molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments? (a) H3O (b) PCl4 (c) SnCl3 (d) BrCl4 (e) ICI3 (f) XeF4 (g) SF2arrow_forwardIdentify if the statement is true or false. If false, explain why the statement is false. a. H for the formation of a bond is always a negative number. b. Because the presence of pi bonds does not influence the geometry of a molecule, the presence of pi bonds does not affect the value of the bond enthalpy between two atoms. c. A hot metal is placed in a calorimeter. The temperature of the water increases. The q value for the metal will be positive and the q value for the water will be negative.arrow_forward1. How can you tell if a compound is covalent? 2. What distinguishes a covalent bond from an ionic bond? 3. How do you know how many valence electrons an atom has? 4. What is electronegativity? Is electronegativity a property of atoms or bonds? 5. What is polarity? Is polarity a property of atoms or bonds? 6. Describe in detail the N-F bond in terms of the relevant electronegativities and polarities. 7. What does VSEPR stand for and how does it allow one to predict the shapes of covalent molecules?arrow_forward
- 1. Mark the following statements (A-E) as true or false A. The halogen bond dissociation energy decreases in the row Cl2 Br2 I2 because of repulsion between the electrons in the lower shells. B. The melting points of the dihalogens increase down the group because the interactions between the dihalogen molecules get stronger C. The electronegativity of the halogens decreases down the group because of the decrease of the electron affinity and the increase of the ionization energy. D. The unusually low bond dissociation energy of the F2 molecule is a direct result of the high electronegativity of fluorine E. The high reactivity of fluorine is in part due to the low bond dissociation energy of the F2 moleculearrow_forwardFor each of the given species:a. Draw its Lewis structure.b. Describe the orbitals used by each carbon atom in bonding and indicate the approximate bond angles.1. H2CO2 2. HCN 3. CC14 4. H2CO3arrow_forwardConsidering the position of the elements in the periodic table and their relative electronegativities and bond polarities, which bond is longest? a. carbon - Oxygen triple bond b. carbon - Oxygen single bond c. carbon - Carbon single bond d. carbon - Carbon double bond e. carbon - Nitrogen triple bond Which bond is the strongest? a. carbon - Nitrogen triple bond b. carbon - Nitrogen double bond c. carbon - Hydrogen bond d. carbon - Carbon triple bond e. carbon - Carbon single bondarrow_forward
- Atom A has 4 valence electrons. Atom Z has 6 valence electrons. For the AZ3-2 ion How many valence electrons are in the structure? b. How many single bonds are in the structure? c. How many double bonds are in the structure? d. How many triple bonds are in the structure? e. How many lone pairs are on the central atom in the structure? f. What is the shape of the structure? g. What are the bond angles of this ion?arrow_forwardL A Moving to another question will save this response. Question 6 For collisions to be successful, reactants must have O a. favorable geometry only ☐ b. sufficient heat of reaction only ☐ c. sufficient kinetic energy and favourable geometry ☐ d. sufficient potential energy only A Moving to another question will save this response. SOLarrow_forward9 A molecule (or ion) has _______ electrons. It is composed of a central atom surrounded by identical atoms. Draw a generic Lewis structure using A for the central atom and X for the outer atoms. Identify at least one molecule and one ion that have the above electron configuration. The electron geometry is The molecular shape is The bond angles are The hybridization of the central atom is MacBook Proarrow_forward
- 12. Acetamide is a colorless, crystalline (sand-like) material. It is used in lacquers, explosives, and soldering flux, and as a stabilizer, plasticizer and solvent. H. -N-H acetamide a. Identify all sigma bonds and pi bonds. What is the total number of each in this molecule? b. Identify the molecular geometry of each central atom c. Draw the 3D Lewis structure for this compoundarrow_forwardDetermine if the structural formula below are acceptable Lewis structures for organic compounds. Point out the problems in cases where structure is invalid. CH3 CH;-N-CH-CH3 ČH3 A. This structure is correct because the valance of Nitrogen is complete. B. This is not a correct Lewis structure because Nitrogen can accommodate more atoms. C. This is not a correct Lewis structure because Nitrogen has a charge if it does not have three bonds. D. This is a correct Lewis structure.arrow_forwardWhich do you predict has the higher boiling point and why? 1.Cl₂ II.Ar Because III. When this substance boils, covalent bonds are overcome. IV. When this substance boils, LDFs are overcome. V. Covalent bonds are stronger than LDFs. VI. The strength of the LDFs depends on the size of the electron cloud. a.l, III, V b.l, IV, VI c.II, IV, VI d.I, III, IV, V e.II, III, V O C Ob O O O d earrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY