
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 13CTQ
A complete Lewis structure must show all nonzero formal charges. Complete each of thefollowing Lewis structures by adding any missing formal charges.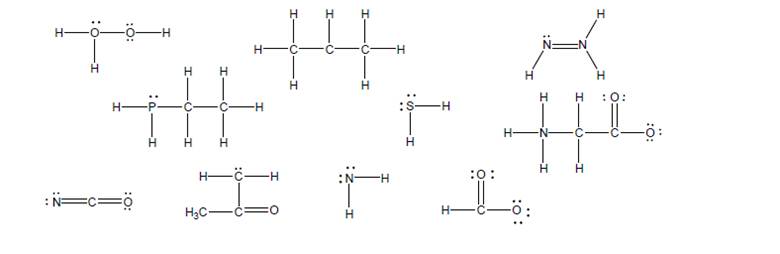
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In the POF, molecule, the P atom is the central atom.
Draw a Lewis diagram of POF, for which all formal charges are equal to zero. How many double bonds are there in the structure that you have drawn?
number of double bonds =
Draw a Lewis diagram in which the octet rule is satisfied on all atoms. What are the formal charges on the following atoms in the structure that you have
drawn?
P
Based on formal charge, which is the best Lewis structure for the molecule?
An incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of zero.
Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.
Draw the Lewis structures for the following four molecules, being sure to show all steps following the methods covered in class. Structures without work shown will be marked incorrect. Also, one of these molecules has resonance structures – for this compound, make sure to include all resonance structures, indicate formal charges for each atom.
SO2 OF2 IF3 NH4+
Consider a molecule where the central atom has one lone pair of electrons and is double-bonded to two other atoms (of a different element). Draw a general diagram of the molecule. Is this molecule likely a polar or nonpolar molecule? Briefly explain your reasoning, using words and the diagram.
Chapter 2 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 2 - Prob. 1CTQCh. 2 - The valence shell of an atom in a legitimate Lewis...Ch. 2 - Prob. 3CTQCh. 2 - Prob. 4CTQCh. 2 - Prob. 5CTQCh. 2 - It is impossible to draw a legitimate Lewis...Ch. 2 - Describe how to calculate the total number of...Ch. 2 - Prob. 8CTQCh. 2 - Prob. 9CTQCh. 2 - Prob. 10CTQ
Ch. 2 - Prob. 11CTQCh. 2 - Prob. 12CTQCh. 2 - A complete Lewis structure must show all nonzero...Ch. 2 - Prob. 14CTQCh. 2 - Prob. 15CTQCh. 2 - Prob. 16CTQCh. 2 - Prob. 17CTQCh. 2 - Prob. 18CTQCh. 2 - Complete the rest of the table for N, O or X by...Ch. 2 - Prob. 20CTQCh. 2 - Prob. 21CTQCh. 2 - Make a checklist that can be used to determine if...Ch. 2 - Prob. 2ECh. 2 - Prob. 3ECh. 2 - Draw the Lewis structure of a neutral molecule...Ch. 2 - Prob. 5ECh. 2 - For each element, predict (and draw a Lewis...Ch. 2 - Predict which of the following species is least...Ch. 2 - The molecules BH3 and SF6 and the ion SO42 exist...Ch. 2 - These are NOTlegitimate Lewisstructures (and...Ch. 2 - Fill in missing formal charges where needed (all...Ch. 2 - Below each structure in the previous question is a...Ch. 2 - Prob. 12ECh. 2 - Carbon monoxide (CO) is an example of an overall...Ch. 2 - Explain why this Lewis structure for CO is not as...Ch. 2 - Prob. 15ECh. 2 - Prob. 16ECh. 2 - Prob. 17ECh. 2 - Prob. 18ECh. 2 - Prob. 19E
Additional Science Textbook Solutions
Find more solutions based on key concepts
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
covered a synthesis of alkynes by a double dehydrohalogenation of dihalides. A student tried to convert trans-2...
Organic Chemistry (9th Edition)
What is the pH range for acidic solutions? For basic solutions?
Introduction to Chemistry
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
Write a Lewis formula for each of the following organic molecules: C2H3Cl (vinyl chloride: starting material fo...
Organic Chemistry - Standalone book
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- the formal charges on all the atoms in the following Lewis diagrams. Which one would best represent bonding in the molecule Cl2O ?arrow_forwardCarbon monoxide (CO) is an example of an overall neutral molecule (netcharge=0) that hasnon-zero formal charges. Draw a Lewis structure of carbon monoxide (CO).arrow_forwardThe molecular ion S3N3 has the cyclic structure All SN bonds are equivalent. (a) Give six equivalent resonance hybrid Lewis diagrams for this molecular ion. (b) Compute the formal charges on all atoms in the molecular ion in each of the six Lewis diagrams. (c) Determine the charge on each atom in the polyatomic ion, assuming that the true distribution of electrons is the average of the six Lewis diagrams arrived at in parts (a) and (b). (d) An advanced calculation suggests that the actual charge resident on each N atom is 0.375 and on each S atom is +0.041 . Show that this result is consistent with the overall +1 charge on the molecular ion.arrow_forward
- An incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of zero. H I H-C-C-N-O | H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.arrow_forwardAn incompicie Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of -1. N-C-C-H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges. H N=c=c-Harrow_forwardcomplete the following lewis structure (draw preferred) and fill in the table. show formal charges on lewis structure.arrow_forward
- Draw the Lewis structures for the following four molecules, being sure to show all steps following the methods covered in class. Structures without work shown will be marked incorrect. Also, one of these molecules has resonance structures – for this compound, make sure to include all resonance structures, indicate formal charges for each atom. SO2 OF2 IF3 NH4+arrow_forwardAn incomplete Lewis structure is shown. The structure only shows the atom and how they are connected. The molecule has a bet charge of -1. Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structure showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-Zero formal charges..arrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of +1. Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.arrow_forward
- Draw the Lewis structures for the following four molecules, Structures without work shown will be marked incorrect. Also, one of these molecules has resonance structures – for this compound, make sure to include all resonance structures, indicate formal charges for each atom. SO2 OF2 IF3 NH4+arrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of -1. H H. H H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formál charges.arrow_forwardA resonance hybrid is a structure that can be depicted by more than one valid Lewis structure. part1: Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part2: Draw the second most important resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part3: Draw the least important resonance contributor for fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized and should include all nonzero formal charges and all nonbonding electrons.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY