
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 13.9, Problem 20P
Interpretation Introduction
Interpretation:
Compounds that are expected to undergo decarboxylation have to be identified.
Concept Introduction:
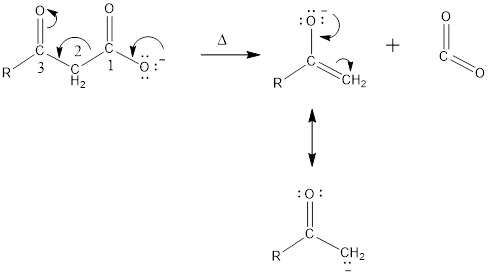
Carbon dioxide can be removed from
Mechanism of decarboxylation is given below,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
With which of the following can ethyl ethanoate not undergo a substitution reaction? *
a-aqueous NaOH
b-CH3OH, H+
c-aqueous NH3
d-CH3COONa
Which of the following compounds will decarboxylate when heated and what will be the organic product?
Which of the following compounds decarboxylates when heated?
Chapter 13 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 13.1 - Identify the most acidic hydrogen in each...Ch. 13.1 - Prob. 2PCh. 13.1 - Prob. 3PCh. 13.1 - Prob. 4PCh. 13.1 - Explain why HO cannot remove a proton from the...Ch. 13.2 - Prob. 6PCh. 13.2 - Prob. 7PCh. 13.3 - Prob. 8PCh. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.4 - Prob. 11PCh. 13.5 - Prob. 12PCh. 13.5 - Prob. 13PCh. 13.6 - Prob. 14PCh. 13.7 - Prob. 16PCh. 13.8 - Prob. 17PCh. 13.8 - Prob. 18PCh. 13.8 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.10 - Propose a mechanism for the formation of...Ch. 13.10 - Prob. 22PCh. 13.10 - a. If the biosynthesis of palmitic acid were...Ch. 13 - Draw the enol tautomers for each of the following...Ch. 13 - Number the following compounds in order from...Ch. 13 - Prob. 26PCh. 13 - Explain why the pKa of a hydrogen bonded to the...Ch. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Using cyclopentanone as the reactant, show the...Ch. 13 - Prob. 35PCh. 13 - Prob. 36PCh. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - Describe how the following compounds can be...Ch. 13 - Prob. 46PCh. 13 - Which would require a higher temperature:...Ch. 13 - Prob. 48PCh. 13 - Propose a mechanism for the following reaction:Ch. 13 - Show how the following compounds could be...Ch. 13 - Prob. 51PCh. 13 - Prob. 52P
Knowledge Booster
Similar questions
- What are the major organic products are formed when the following compounds react with methylmagnesium bromide (CH3MgBr), followed by the addition of dilute acid? propanal 2-pentanonearrow_forwardEsterification of Carboxylic Acids Provide the reactions (chemical equations) on esterification of the following acids: Ethanoic acid Butanoic acid Benzoic acid Acetic acid + methanol Acetic acid + ethanol Salicylic acid + methanol Acetic acid + benzyl alcoholarrow_forwardPredict the products formed when cyclohexanone reacts with the following reagents.ethylene glycol and p-toluenesulfonic acidarrow_forward
- Which of the following reactants would lead to an ester product as the major organic product? ? CH₂=CH2 CH3OH CH3CH3 CH3COOH + Esterarrow_forwardState the Reactions of Aldehydes and Ketones with Grignard Reagents:arrow_forwardWhich of the following reactants would lead to an ester product as the major organic product? ? CH₂=CH₂ CH3OH CH3CH3 CH3COOH + R R Esterarrow_forward
- Hydration of aldehydes and ketones can be catalyzed by acid or base. Bases catalyze hydration by: protonating the carbonyl oxygen making the carbonyl group more electrophilic employing hydroxide ion, which is a better nucleophile than water making the carbonyl group less electrophilic shifting the equilibrium position of the reaction to favor productsarrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l)CH3CH2CH2CO2H(l)+CH2CH3OH(l)⟶H+CH3CH2CH2CO2CH2CH3(l)+H2O(l) A chemist ran the reaction and obtained 5.40 g of ethyl butyrate. What was the percent yield, The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.45g of butanoic acid and excess ethanol?arrow_forwardWhich will undergo hydrolysis to form a carboxylic acid?arrow_forward
- Which of the following is the condensation product formed from two moles of Acetophenone (C6H5COCH3) in the presence of base?arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.45 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. A chemist ran the reaction and obtained 5.50 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 8.45 gg of butanoic acid and excess…arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning