
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.35SP
Predict the approximate chemical shifts of the protons in the following compounds.
- a. benzene
- b. cyclohexane
- c. CH3 — O — CH2CH2CH2Cl
- d. CH3CH2 — C = C — H
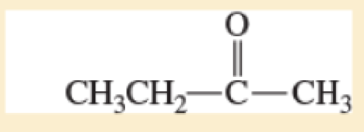
- e. (CH3)2CH — O — CH2CH2OH
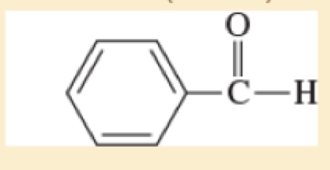
- f. CH3 — CH = CH — CHO
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Rank Ha, Hb, and Hc in order of increasing chemical shift.
Hb
Hc
H2
H3C
CH3
Hd
OCH2CH3
Hb Ha
На
Не
а.
b.
Which of the following frequencies corresponds to the highest ener
O 9.12 x 1012-1
O 3.20 x 10%s1
O 8.50 x 1020s!
O 3.00 x 103
S.
O 4.12 x 10 s 1
5. L-dopa is used in the treatment of Parkinson's disease. Identify the four most acidic
protons and arrange them in order of increasing acidity.
:O:
g
HO:
:OH
6. Coumarins are a class of natural products produced by plants and animals. It has a wide
variety of applications in cosmetics, food industry and fluorescent laser dyes. In the
¹HNMR spectrum of coumarin derivate shown below, the two signals farthest downfield
are at 7.38 and 8.42 pm. Identify the two protons that most likely give rise to these
signals and justify your answer using resonance structures.
:O:
Chapter 13 Solutions
Organic Chemistry (9th Edition)
Ch. 13.5A - In a 300-MHz spectrometer, the protons in...Ch. 13.5B - Prob. 13.2PCh. 13.6 - Determine the number of different kinds of protons...Ch. 13.6 - Prob. 13.4PCh. 13.7 - Draw the integral trace expected for the NMR...Ch. 13.7 - Prob. 13.6PCh. 13.8C - Draw the NMR spectra you would expect for the...Ch. 13.8D - Draw the NMR spectra you expect for the following...Ch. 13.8D - a. Assign protons to the peaks in the NMR spectrum...Ch. 13.8D - Prob. 13.10P
Ch. 13.8D - Two spectra are shown. Propose a structure that...Ch. 13.9 - Prob. 13.12PCh. 13.9 - The spectrum of trans-hex-2-enoic acid follows. a....Ch. 13.9 - Prob. 13.14PCh. 13.9 - Prob. 13.15PCh. 13.10 - Prob. 13.16PCh. 13.10 - If the imaginary replacement of either of two...Ch. 13.10 - Predict the theoretical number of different NMR...Ch. 13.11B - Prob. 13.19PCh. 13.11B - Prob. 13.20PCh. 13.11B - Prob. 13.21PCh. 13.11B - Prob. 13.22PCh. 13.11B - Prob. 13.23PCh. 13.11B - Prob. 13.24PCh. 13.12E - Draw the expected broadband-decoupled 13 C N M R...Ch. 13.12E - a. Show which carbon atoms correspond with which...Ch. 13.12E - Repeat Problem13-25, sketching the...Ch. 13.12F - Prob. 13.28PCh. 13.13 - A bottle of allyl bromide was found to contain a...Ch. 13.13 - A laboratory student was converting cyclohexanol...Ch. 13.14 - Sets of spectra are given for two compounds. For...Ch. 13 - An unknown compound has the molecular formula C 9...Ch. 13 - Prob. 13.34SPCh. 13 - Predict the approximate chemical shifts of the...Ch. 13 - Prob. 13.36SPCh. 13 - Prob. 13.37SPCh. 13 - Prob. 13.38SPCh. 13 - Prob. 13.39SPCh. 13 - Prob. 13.40SPCh. 13 - For each compound shown below. 1. sketch the 13 C...Ch. 13 - Prob. 13.42SPCh. 13 - Prob. 13.43SPCh. 13 - Prob. 13.44SPCh. 13 - Prob. 13.45SPCh. 13 - Prob. 13.46SPCh. 13 - A compound was isolated as a minor constituent in...Ch. 13 - Prob. 13.48SPCh. 13 - The three isomers of dimethylbenzene are commonly...Ch. 13 - a. Draw all six isomers of formula C 4 H 8...Ch. 13 - Prob. 13.51SPCh. 13 - Hexamethylbenzene undergoes free-radical...Ch. 13 - Each of these four structures has molecular...Ch. 13 - Prob. 13.54SPCh. 13 - Phenyl Grignard reagent adds to 2-methylpropanal...Ch. 13 - Prob. 13.56SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Shown below is the Proton NMR of a hydrocarbon of the formula C11H16. Given the number of hydrogen atoms represented by each of the 3 integrals, what is the structure of the compound?arrow_forwardWhat do you understand by estimation? Justify that using higher values of Cl gives better estimations.arrow_forwardEach of the two IR spectra are one of the following benzene derivatives: benzaldehyde, benzoic acid, benzyl alcohol, methyl benzoate, and benzamide. What derivative is represented by each graph?arrow_forward
- CH2=CH-C-O-CH3 C3 C2 C1 Cme Which order indicates the correct order of carbon chemical shift of the four carbons of the compound ○ A. C₂ < C3 C1 < Cme ○ C₁< B. C3 < C2 Cme О с. Cme V C3 < C1 C2 Varrow_forwardWhich is TRUE for spectroscopy of a mixture? A. The absorbance for a mixture at a particular wavelength is the sum of the absorbances for the components that absorb at the particular wavelength. B. Spectrophotometers can differentiate between mixture components that absorb at the same wavelength. C. Each component in a mixture has the same molar absorptivity at the same wavelength. D. The concentration for each component in a mixture is easily calculated by least squares for guesses of each component when the individual spectra are well resolved.arrow_forwardDecreasing order of chemical shift of H?arrow_forward
- 5. A sample of a red dye and a sample of a blue dye both have the same concentration. Their Absorbance is measured using the same spectrophotometer. At a particular wavelength, will both samples give the same Absorbance reading? Why or why not?arrow_forward(How do the signals for methyl fluoride look like in the proton and the carbon NMR Spectrum?) the splitting caused by atoms other than carbon and hydrogen atoms Predict the number of lines observed in the proton spectrum. Predict the number of lines observed in the carbon spectrum. Justify your answer for the proton spectrum. Justify your answer for the carbon spectrum.arrow_forwardHow to determine equatorial vs axial in NMRarrow_forward
- 1. how aas work? 2. how uv-vis work? 3. how nmr work? 4. how infrared spectroscopy work? 5. how molecular spectroscopy work?arrow_forward• Predict chemical shifts for all of the protons in the molecule below. 0 Cl OH HDarrow_forward5:58 .ull LTE O AA A bcps.schoology.com 3 of 3 Which wave pattern has the highest pitch? If necessary, review the adapted reading "How do patterns found in sound waves affect how sounds are heard and felt? FIGURE I A B Sound wave A has the highest pitch because it has the smallest distance from the center point to the crest. O Sound wave B has the highest pitch because it has the longest wavelength. Sound wave C has the highest pitch because it has the highest amplitude (distance from the center point to the crest). All the sound waves have the same pitch because they have the same frequency and wavelength. MReview >arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY