
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30.8, Problem 8P
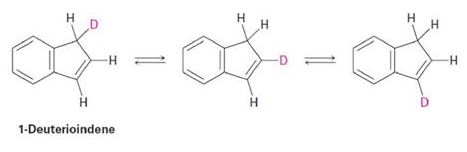
Propose a mechanism to account for the fact that heating 1-deuterioindene scrambles the isotope label to all three positions on the five-membered ring:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Dehydrohalogenation of 1-chloro-1-methylcyclopropane affords two alkenes (A and B) as products. Explain why A is the major product despite the fact that it contains the less substituted double bond.
Bromine adds to cis- and trans-2-butene to give different diastereomers of 2,3-dibromobutane. What does this say about the mode of addition of bromine to thisalkene?
Addition of HBr to allene (CH2=C=CH2) forms 2-bromoprop-1-ene ratherthan 3-bromoprop-1-ene, even though 3-bromoprop-1-ene is formed froman allylic carbocation. Considering the arrangement of orbitals in theallene reactant, explain this result.
Chapter 30 Solutions
Organic Chemistry
Ch. 30.1 - Prob. 1PCh. 30.3 - Prob. 2PCh. 30.3 - Prob. 3PCh. 30.4 - Prob. 4PCh. 30.6 - What stereochemistry would you expect for the...Ch. 30.6 - Prob. 6PCh. 30.7 - Prob. 7PCh. 30.8 - Propose a mechanism to account for the fact that...Ch. 30.8 - When a 2, 6-disubstituted allyl phenyl ether is...Ch. 30.9 - Prob. 10P
Ch. 30.SE - Predict the product obtained when the following...Ch. 30.SE - Prob. 12VCCh. 30.SE - The following rearrangement of N-allyl-N,...Ch. 30.SE - Plastic photochromic sunglasses are based on the...Ch. 30.SE - Prob. 15MPCh. 30.SE - Prob. 16MPCh. 30.SE - Prob. 17MPCh. 30.SE - Prob. 18APCh. 30.SE - Prob. 19APCh. 30.SE - Prob. 20APCh. 30.SE - Prob. 21APCh. 30.SE - Prob. 22APCh. 30.SE - Prob. 23APCh. 30.SE - Prob. 24APCh. 30.SE - Prob. 25APCh. 30.SE - Prob. 26APCh. 30.SE - Prob. 27APCh. 30.SE - Prob. 28APCh. 30.SE - Propose a pericyclic mechanism to account for the...Ch. 30.SE - Prob. 30APCh. 30.SE - Prob. 31APCh. 30.SE - Prob. 32APCh. 30.SE - Prob. 33APCh. 30.SE - Bicyclohexadiene, also known as Dewar benzene, is...Ch. 30.SE - Prob. 35APCh. 30.SE - Prob. 36APCh. 30.SE - The 1H NMR spectrum of bullvalene at 100 C...Ch. 30.SE - Prob. 38APCh. 30.SE - Prob. 39APCh. 30.SE - Prob. 40APCh. 30.SE - In light of your answer to Problem 30-40, explain...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -What of the following statements is false about cyclopentadienyl anion? A) It is aromatic B) It has 5 p molecular orbitals C) It has three full p bonding molecular orbitals D) It has one empty p anti-bonding molecular orbital E) It has two empty p anti-bonding molecular orbital 6. What is the correct IUPAC name of the following compound? A) 1-bromo-3-chloro-5-isobutyl-4-methylbenzene B) 5-bromo-1-chloro-3-sec-propyl-2-methylbenzene C) 5-bromo-1-sec-butyl-3-chloro-2-methylbenzene Br D) 1-bromo-3-sec-butyl-5-chloro-4-methylbenzene E) 5-bromo-1-chloro-3-isobutyl-2-methylbenzene II. Draw the structure for the major organic product of each of the following reaction: 7. CN + -> NC Show strerochemistry if necessary HBr 8. (1 equivalent) (45°C) Show strerochemistnuarrow_forwardDraw and name all stereoisomers of 3-chlorohepta-2,4-diene(a) using the cis-trans nomenclature.(b) using the E-Z nomenclaturearrow_forwardShow how to convert cyclopentene into cis-1,2-Cyclopentanediolcompoundarrow_forward
- 1. In the lecture on the reactions of alkenes, we studied the reaction of alkenes with N- Bromosuccinimide (NBS), which went through a mechanism involving an allylic radical (the radical is a carbon attached to a carbon involved in a л bond). What if there were a conjugated system? Draw, using curved arrows, the three resonance forms of cyclohexadienyl radical:arrow_forwardprovide a systematic [IUPAC] name for each of the following C ΟΗarrow_forward7. Which of the following statements regarding the cyclopropenyl anion is correct? and why?a) It is aromatic.b) It is not aromatic.c) It obeys Hückel’s rule.d) It undergoes reactions characteristic of benzene.e) It has a closed shell of 6 pi electrons.arrow_forward
- Explain why heats of hydrogenation cannot be used to determine the relative stability of 2-methylpent-2-ene and 3-methylpent-1-ene. A, B, and C, each subjected to hydrogenation. The number of rings and π bonds refers to the reactant (A, B, or C) prior to hydrogenation.arrow_forward(a) Draw all stereoisomers formed by monochlorination of the cis and trans isomers of 1,2-dimethylcyclobutane drawn below. (b) How many constitutional isomers are formed in each reaction? (c) Label any pairs of enantiomers formed.arrow_forwardCompound A, C₂H16 reacts with 1 molar equivalent(s) of hydrogen on catalytic hydrogenation. A undergoes reaction with ozone, followed by Zn treatment, to give: O CH3 O || CH3CCH₂CCH₂CCH3 CH3 Compound A Propose a structure for A. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forward
- Identify two alkenes that react with HBr to form 1-bromo-1-methylcyclohexane without undergoing a carbocation rearrangement.arrow_forward1) Provide the name for each structure shown. NO ₂ 2) Draw the structure of the molecule with the following name: a) (1R, 3S)-Cyclopent-4-ene-1,3-diol b) (2R, 3Z)-3-Chloro-2phenylhex-3-enal c) (2E,4Z)-Hepta-2,4,6-trienoic acid d) (3R, 4E)-3-isopropyl-6-oxohex-4-enenitrile H₂C NH CH3 CH3 H₂N CH3arrow_forwardEthylene oxide reacts readily with HO- because of the strain in the three-membered ring. Explain why cyclopropane, a compound with approximately the same amount of strain, does not react with HO-.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY