
PRACTICE PROBLEM 21.1
For each of the following complexes, determine the oxidation state of the metal and the total number of valence electrons it possesses.
(a)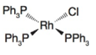
(b)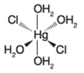
(c)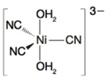
Interpretation:
The oxidation state of a metal and the total number of valence electrons in metals in the complexes is to be determined.
Concept introduction:
The oxidation state of a metal in a complex is the charge on the metal that would be there even if all the anionic ligands and counter ions were removed.
The total number of valence electrons of a metal in a complex is obtained by the following formula:
Total number of valence electrons of metal in complex =
Here,
Answer to Problem 1PP
Solution:
(a)
Oxidation state of Rh is
(b)
Oxidation state of Hg is
(c)
Oxidation state of Ni is
Explanation of Solution
Given information:
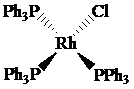
The oxidation state of rhodium is
Charge on rhodium is as follows:
Here,
Now, d-electrons in rhodium are
Total number of valence electrons of metal in complex =
Here,
For rhodium, the total number of valence electrons in the complex
Thus, the number of valence electrons in rhodium is
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Organic Chemistry
Chemistry
Chemistry: The Molecular Nature of Matter
Fundamentals of Heat and Mass Transfer
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry For Changing Times (14th Edition)
- How many bonds could each of the following chelating ligands form with a metal ion? (a) acetylacetone, a common ligand in organometallic catalysts CH3-C-CH2-C-CH3 (b) diethylenetriamine, used in a variety of industrial processes NH2-CH2-CH2-NH-CH2-CH2-NH2 (c) salen, a common ligand for chiral organometallic catalysts EN N: -OH Но-arrow_forwardArrange the following complexes in order of increasing stabilityarrow_forward(5) Give the hapticity of the ligands identified with an arrow (using the correct terminology) in the following complexes and give the electron count and formal oxidation state for the iron complex: (a) (c) CH3 ос (b)arrow_forward
- Determine if each of the following complexes exhibits geometric isomerism. If geometric isomers exist, determine how many there are. (a) square-planar [IrCl2(PH3)2]-arrow_forward(a) Draw all the possible isomers of [Fe(NH;);Br3] and for each isomer clearly indicate the relationship between equivalent ligands. (b) What is the oxidation state of the iron ion in [Fe(NH3)3Br3]? (c) The magnetic moment of [Fe(NH:)3Br3] is 5.92 BM. Specify if the complex is low spin or high spin and explain your answer using an appropriate d-orbital splitting diagram.arrow_forwardSuppose that you want to determine whether a ligand X is a weak-field or strong-field ligand. Would you synthesize an octahedral complex of ligand X with Cr3+ or Co3+? Explain your reasoning and illustrate with relevant appropriate crystal-field energy diagrams for each complex.arrow_forward
- (III) Draw the structure of the following coordination compounds. (a) Pentammineiron(III)-μ-hydroxopentammineiron(III) bromide (b) Λ-Tris(oxalato)vanadate(III) ionarrow_forward(a) (i) State the oxidation state and d configuration for iron in the complex anion [Fe(CN) and the complex cation [Fe(OH)]³¹. (ii) Draw crystal field d-orbital energy level diagrams for both complexes in part (a)(1). (iii) State the two factors that affect the intensity of d-d transitions in transition metal complexes. part falli), what determines thearrow_forward(a) Using Valence bond theory explain the geometry and magnetic behaviour by [Cr(NH3)6]3+ . (At. no. Cr = 24)(b) Write the IUPAC name of ionization isomer of [Ni(NH3)3NO3]Cl.arrow_forward
- flouroquinolones like ciprofloxacin are antibiotics with broad antibacterial spectrum widely used nowadays to treat bacterial infections. unfortunately, they form water insoluble complexes in the GIT when taken orally together with divalent metal supplements like zinc and iron as sulfate salts respectively [ both in the second oxidation state ] a. draw the structure of the resulted metals complexes with ciprofloxacin b. expect the electronic configuration showing the complexation orbitals and sharing for each metal case c. how many coordination covalent bond are expected form such complexation and the type of hybridization expected d. what would be the influence of such complexation on the bioavailability of both the metals and the antibiotic e. which functional groups in the ciprofloxacin structure are expected to be the ligands in the resulted complexes. f. which type of chelating agent ciprofloxacine would be expected in these cases.arrow_forwardThe SCN – ligand is an ambidentate ligand. When it is S-bound, it has the name thiocyanate, and the M–S–C bond angle is bent. When it is N-bound, it has the name isothiocyanate, and the M–N–C bond angle is ~180 ̊. (a) Draw Lewis dot structures for M–NCS and M–SCN that explain the observed geometries. (b) Predict the structures of the metal-ligand bonding when SCN– reacts with Fe 2+ to form the bright red Fe(SCN)2+ ion. (c) Predict the method of bonding when Cr3+ reacts with the SCN – ligandarrow_forwardName the wo compl With the ligand field theory give a qualitative energetic scheme of the d orbitals in an octahedral environment and indicate the valence electron repartition for the metal in the [PtClo] complex, assuming that the chloride ions give rise to a strong ligand field. ligands along z from the central atom toarrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
