
Basic Chemistry
6th Edition
ISBN: 9780134878119
Author: Timberlake, Karen C. , William
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 89UTC
The chapter sections to review are shown in parentheses at the end of each problem.
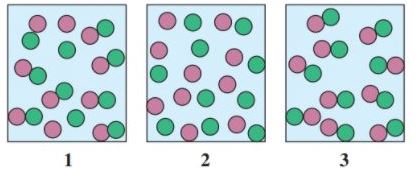
12.89 Select the diagram that represents the solution formed by a solute  that is
that is
a. nonelectrolyte
b. weak electrolyte
c. strong electrolyte
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
9.56 Calculate the final concentration of each of the following:
a. 1.0 L of a 4.0 M HNO, solution is added to water so that
the final volume is 8.0 L.
b. Water is added to 0.25 L of a 6.0 M NaF solution to make
2.0 L of a diluted NaF solution.
c. A 50.0-mL sample of an 8.0% (m/v) KBr solution is
diluted with water so that the final volume is 200.0 mL.
d. A 5.0-mL sample of a 50.0% (m/v) acetic acid (HC,H,O2)
solution is added to water to give a final volume of 25 mL.
How many mL of 0.4M solution contains 0.200 mol of solute? (3 SF)
8.79 What is the boiling point of a solution that contains each of the
following quantities of solute in 1.00 kg of water?
a. 3.0 mol of fructose molecules
b. 1.2 mol of KI
c. 1.5 mol of Na PO,
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Page 5. Calculate the molarity for each of the following solutions. (6 points) a. Dissolving 45.1 moles of KCl into 1005 ml of solution. b. Dissolving 45.1 grams of KCa into 115 mL of solution.arrow_forward8.59 How would you use a 250-mL volumetric flask to prepare each of the following solutions? a. 4.8% (w/v) acetic acid in water b. 22% (v/v) ethyl acetate in water C. 2.5 M NaCI solutionarrow_forwardanchPad G Google O Hudl O Football Pics BIM - 1st Art 1- 2nd A WoHistory 4th AAlg2-5th A English - 6th Chem 4. 6. 7 8. 9. 10 What is the molarity of a solution containing 15.0 g of sodium hydroxide dissolved in 1.00 L of solution? Molar mass of NaOH is 40.0 grams/mole. SHOW YOUR WORK 8 10 Show Your Work 0.500 M moles 0.400 M M= 0.200 M Liter 0.374 M What is the total number of moles of NaCl(s) needed to make 1.5 liters of a 2.0 M NaCl solution? SHOW YOUR WORK 9. 10 Show Your Work 1.3 mol 5 2,269 MAY 12arrow_forward
- 9.44 For each of the following solutions, calculate the: a. grams of 2.0% (m/m) NaCl solution that contains 7.50 g of NaCl b. milliliters of 25% (m/v) NaF solution that contains 4.0 g of NaF c. milliliters of 8.0% (v/v) ethanol solution that contains 20.0 mL of ethanolarrow_forwardConcentration Units Continued Molarity (M) moles of solute M = liters of solution Molality (m) moles of solute m 3D mass of solvent (kg) 12.3 (Other two equations at the end) (All 4 equations in the notes) CH 14 HANDOUT: MOLARITY, MOLALITY , DILUTION, AND MOLE FRACTION: Name: 1. Find the molarity of a solution that has 26.5 grams of salt (NaCI) in 345 ml of total solution. (Hint: change grams to moles using molar mass of NaCl AND change ml to Liters using 1000 ml = 1 Liter) 2. Find the molarity of 4.3 moles of sugar in 1.545 liters of water solution. 3. Find the molarity of a solution with 1.57 grams of CaCl2 in 1344 ml of solution.arrow_forwardCHEM 120: Unit 4 - Problems from Text: Chapter 9: Solutions Name: 9.9 PRACTICE PROBLEMS 9.1 Solutions 9.1 Identify the solute and the solvent in each solution composed of the following: Solute 9.3 9.11 a) b) c) Clinical Applications 9.5 Water is a polar solvent and carbon tetrachloride (CCI) is a nonpolar solvent. In which solvent is each of the following, which is found or used in the body, more likely to be soluble? 10.0 g of NaCl and 100.0 g of H₂O 50.0 mL of ethanol, C,H,O, and 10.0 mL of H₂O 0.20 L of O₂ and 0.80 L of N₂ Describe the formation of an aqueous KI solution, when solid KI dissolves in water. CaCO, (calcium supplement), ionic a) b) retinol (vitamin A), nonpolar c) sucrose (table sugar), polar d) cholesterol (lipid), nonpolar PRACTICE PROBLEMS 9.2 Electrolytes and Nonelectrolytes 9.7 KF is a strong electrolyte, and HF is a weak electrolyte. How is the solution of KF different from that of HF? a) KCI Write a balanced equation for the dissociation of each of the following…arrow_forward
- At 20°C, the solubility of potassium carbonate, K2CO3, is 110.3 g/100. mL of water. In the laboratory, a student mixes 215 g of K2CO3 with 175. g of H2O at a temperature of 20°C. (9.3) a) How much of the K2CO3 will dissolve? b) Is the solution saturated or unsaturated? c) What is the mass, in grams, of any solid K2CO3 left undissolved on the bottom of the container?arrow_forward(14.6) Which of the following aqueous solution has the highest boiling point? O 1.00 M NaCl O 1.00 M Ca(NO3)2 O 1.00 M C6H1206 O 1.00 M NaNO3arrow_forwardCalculate the molarity of 0.350 mol of Na, S in 1.65 L of solution. molarity: M Calculate the molarity of 28.5 g of MgS in 823 mL of solution. molarity: about us careers privacy policy terms of use contact us help PrtScn PgUp F11 PgDn F12 DII F5 Home End F10 F4 F6 F7 24 & 6 7. 8. 9- R Y U 1Oarrow_forward
- One liter of Jell-O contains 78g of powder. What is the % concentration of Jell-O? a. 78% (v/v) b. 7.8% (v/v) c. 78% (w/v) d. 7.8% (w/v)arrow_forwardQuestion: A solution was prepared by dissolving 25 grams of fruit sugar in sufficient quantity of water to make 100 grams of solution. 1. What is the %W/W of the solution? 2. What is the molecular weight of the solute? 3. What is the mole of solute? Your answer here will be used for the next item(s). 4. What is the mole of solution? Your answer here will be used for the next item(s). 5. What is the mole percent of solute? 6. What is the molarity of the solution?arrow_forwardAnswer the following problems. Points are distributed as follows: Given: Unknown: Formula: Solution: Final Answer What is the molality of a solution containing 100 grams sucrose (342g/mol) in 100.0 grams water? 2. If 150.0 g of KCI (74.55 g/mol) is dissolved in water to make 250 mL solution, what is the molarity of the solution? 3. 1. What is the molarity of 500-mL solution which contains 40 g urea (CH«N2O)?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY