Thalidomide exists in two enantiomeric isomers as shown below. ((R) N۰۰۰ ((S) If a solution is prepared by adding a 50:50 ratio of two enantiomers what will be the expected observed rotation? Explain your answer in 1-2 sentences.
Q: Which of the protons in the following molecule has the highest chemical shift H3C-O-CH₂-CH₂-C-H iv
A: Aldehydic protons have the highest chemical shift in NMR spectroscopy due to the electron…
Q: which pair of structures below contain valid resonace structures of a single Compond? All love pairs…
A:
Q: Which of the following statements is correct with regards to paracetamol? Dichloromethane is a good…
A: Recrystallization is a commonly used purification technique for solids, including paracetamol. In…
Q: Calculate the initial [S₂O32] on the paper ([S₂O3²-]o), assuming the amount of S₂O3²- you added…
A: To find out the initial concentration of S2O32- on the paper, assuming that the amount of S2O32-…
Q: 8. Give the major organic product(s) of each of the following reactions. If none is predicted, write…
A: According to Q&A guidelines of Bartleby, we are supposed to answer only the first question out…
Q: 2,3,4-trimethyl-1-hexene CH3 ICHCH CHO CH2 CH3 1 CH3 Снз CH₂=
A: This question is related to IUPAC (International union of pure and applied chemistry) nomenclature.…
Q: Calculate the activation energy, in kJ/mol, for a given reaction if the rate constant increases 7).…
A: Given data K1 = 2.50 s-1 at T1 = 22 °C = 295 K K2 = 12.5 s-1 at T2 = 35 °C = 308 K We know…
Q: What is the name of the molecule shown in the below image?
A: Since,Rule of IUPAC-1) Longest chain as parent chain.2) Numbering start from those side where more…
Q: How many L NaOH of a 0.075 M NaOH solution is needed to completely neutralize 100.0 mL of a 0.250 M…
A: Given, volume of H2SO4 solution = 100.0 mL = 100.0 mL x ( 1 L / 1000 mL) = 0.100 L molarity of H2SO4…
Q: 2. What is the relationship between these two molecules o a. Identical b. Constitutional isomers c.…
A:
Q: Temperature, °C Estimating from the heating curve show, what is the approximate melting point of the…
A: When a substance undergo phase change (solid to liquid or liquid to gas ) the energy involved in a…
Q: 17. Draw a structure for each alcohol below: isopropyl alcohol methanol 2,3-propanetriol
A: Alcohol is a psychoactive substance that is found in many beverages and is commonly referred to as…
Q: 11. Calculate the change in internal energy for the combustion of 1.0 mol of propene given that the…
A: The change in enthalpy is given to be -2058 kJ. The number of moles of propene combusted is 1.0 mol.…
Q: 1. What is the stereochemical relationship between compounds 2-9 and compound 1? sif- H CHCH 3 Me Et…
A:
Q: Penicillin is hydrolyzed and thereby rendered inactive by penicillinase, an enzyme present in some…
A: “Since you have posted a question with multiple sub-parts, we will solve the first three subparts…
Q: Balance the equation 235U+n→?+Zr+2n. O Te None of the above O 135 Sn 50 O Sn 50 O Te
A:
Q: Nuclei with the same number of neutrons but different mass numbers are called isotones. Write the…
A: Nuclei with the same number of neutrons but different mass numbers are called isotones. Mass number…
Q: 9.18 Calculate the value with which you divide molar magnetization Mot in emu/mol in order to get…
A: Molar magnetization(Mmol) in emu/mol is divided by 9.27400968 x 10^-24 to get the amount of bohr…
Q: 13. Draw all potential constitutional isomers and stereoisomers for the compound below; AND point…
A: Constitutional isomers :- It is also known as structural isomers. The isomers which have same…
Q: A substance is a brittle crystal that conducts electricity in molten liquid state only. Which type…
A: Given : different types of crystal
Q: The synthesis of nitrogen monoxide is first order in [N₂] according to the reaction below, and has a…
A: Given : reaction is first order Time = 66 minutes Initial concentration of nitrogen = 2.95…
Q: Why can’t the CHCl3/AlCl3 test be performed in H2O or CH3OH?
A: The choice of solvent for a reaction is important as it can have a significant impact on the…
Q: 10. Among the following anions, which one could not exist (to any significant extent) in water? B)…
A: Answer: When an acid loses its H+ ion then the specie formed is its conjugate base and it has the…
Q: 1. Provide an arrow-pushing mechanism for the following reactions: NaCN EtOH/H-O b. c. d. H Br Br Br…
A: for SN1 reaction :- Reactivity order of alkyl halide; 3° alkyl halide > 2° alkyl halide > 1°…
Q: Solid sodium azide, NaN3 is used in automobiles to rapidly fill the airbags after a collision has…
A:
Q: Determine the number of atoms of C in 49.3 grams of C6H12O6.
A: Given, mass of C6H12O6 = 49.3 g we know, molar mass of C6H12O6 = 180.16 g/mol => moles of…
Q: Figure 3-7 100 IT A 60 0. 100 1 0 4800 100 80 3000 W тари B 1000 20 0 4000 IR2017-91129TM 3000 2000…
A: The IR spectrum is used for the confirmation or detection of functional groups present in the…
Q: a. b. C. conc. HCI heat Br ( =)
A: Given are organic reactions. First reaction given is hydrochlorination reaction of alkene.
Q: Please provide structures that best agree with the information provided below. A. Molecular Formula:…
A: Given two unknown molecules with molecular formula
Q: How much heat is absorbed or released when 40.00 g of NH3(g) reacts in the presence of excess O2(g)…
A: According to the question, The mass of the ammonia gas is given by =40.00 g The molar mass of the…
Q: be care, you T Calculate the volume of gas in liters produced at 470. C and 1.00 atm when 348 grams…
A: According to the question, The mass of the ammonium nitrate is given by = m = 348 grams The…
Q: Consider the reaction: N2O4 (g) ⟷ 2 NO2 (g) KP = 0.36 (at 100°C) The reaction above is carried out…
A:
Q: (a) Determine whether this hydrogen peroxide reaction is first-order or second-order by first…
A: A first-order reaction is one in which the rate of reaction depends only on one reactant…
Q: The reaction A + 2B → C has the rate law rate = k[A][B]. By what factor does the rate of reaction…
A: Given, A + 2B ----> C rate = k[A][B] By what factor does the rate of the reaction increase when…
Q: Three samples of three different gases are listed in the table below. All the samples contain…
A: Introduction The ideal gas equation, also known as the ideal gas law, states that PV = nRTwhere, P…
Q: Melting point is used in the recrystallization experiment to assess purity of solid samples.…
A: The melting point of a substance is the temperature at which it changes from a solid to a liquid…
Q: Use the observation in the first column to answer the question in the second column. observation The…
A: In the given question, there are three subparts. These subparts are regarding the relation between…
Q: The following 'H NMR spectrum is shown in two questions. "nmrsim presentation 1 1 C:Bruker…
A: In case of 1H-NMR spectrum, no. of peaks obtained is dependent on the no. of different types of…
Q: An exothermic reaction is one where none of these heat is transferred from a system into the…
A: we have to select the correct statement for an exothermic reaction
Q: Show what Grignard reagent and what carbonyl compound you would start with to prepare the alcohol…
A: Grignard reagent is an alkyl magnesium halide. The general formula of Grignard reagent is RMgX. R =…
Q: The formula CH3C=CCH3 represents an alkyne. an alkane. an aromatic compound. O a cycloalkane. O an…
A: Hydrocarbons are made from only hydrogen and carbon these are may be open chain, cyclic ring,…
Q: 2. Consider the following reactions: A -> B (1) B + M -> C (2) Assume M is present in great…
A: The following is a step by step explanation of the rate equations and time-dependent variation in…
Q: - Name the following compounds according to IUPAC rules: (1) (2) (3) (4) J
A: Note: Since you have posted a question with multiple sub-parts, we will provide the solution only to…
Q: When a small amount of water is added to t-butyl, what happens to the cooling curve for this new…
A: Colligative properties are properties of a solution that depend on the concentration of solute…
Q: Which of the following molecules exhibit hydrogen bonding? Write "yes" if it does or "no" otherwise.…
A:
Q: The molar heat of vaporization of water is 42 kJ/mol. How much energy is released by the…
A:
Q: 2) Consider the following reaction and the data below: 2NO +2H₂ → N₂ + 2H₂O [H₂], M 0.100 0.185…
A: a) we have to find the order of each reactant
Q: Activity C: Visual Analysis: ct Na Water molecule Crystal of NaCl Hydrated sodium ion Hydrated…
A: (1) In figure first clearly given that around sodium ion oxygen atoms of water molecules arranged…
Q: Grams of Solute (g) F 8 60 A 12 50 10 20 30 40 50 70 80 Temperature (c²) 90 100
A: The given graph shows the patterns of solubility amount of various solute (i.e. salts, gases) with…
Q: Consider the following rate law: Rate = K[B] Which of the following equations could be used to find…
A: As we know the sum of the powers of the concentration terms occur in the rate law expression is…

Given that thalidomide exists in two enantiomeric isomers as shown below
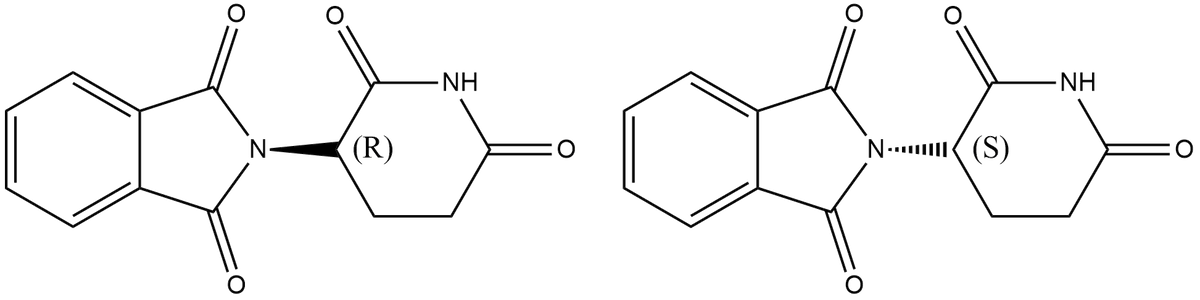
A solution is prepared by mixing 50:50 ratio of two enantiomers.
The expected observed rotation is
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- The specific rotation, [a]p, for (-)-2-butanol is +14. What is the observed rotation for a solution of 2.5 g of (-)-2-butanol in 10 mL of water in a sample tube having a pathlength of 10 cm? degrees. The observed rotation of a solution of 0.75 g of a compound in 10 mL of water is -4.6 degrees. If the pathlength is 10 cm, what is the specific rotation of the compound?How many stereocenters are present in diosgenin (structure below)? Me HO Me H I Me H Me I..(9) How many chiral centers are present in a molecule of 1,3-dimethylcyclohexane? (C) 2 (D) 3 1 (A) O (10) What is the percent composition of a mixture of (S)-(+)-2-butanol, [a] = +13.52°, and (R)-(-)-2-butanol, [a] = -13.52°, with a specific rotation [a]D = -6.76° (A) 75%R 25%S (B) 25%R 75%S (C) 50%R 50%S 67%R 33%S
- Note: enantiomers have similar physical properties (mp, density, solubility), and same [a], but with different signs ( - or +). Practice: 1.2 gm sample of cocaine, [a], = -16º, was dissolved in 7.5 mL of chloroform and placed in a sample tube having a pathlength of 5 cm. what was the observed rotation in degrees?5. Consider the following pairs of structures. Identify the relationship between them (enantiomers, diastereomers, or identical compounds. HOH2C H OH H OH HO H Br H3C Н H H₂CH₂C CI Br H CHO CH3 I CH3 OH OHC Н H OH H OH Bell!! H3C, HO H Н.С H CH₂CH3 CH3 CI HỌ,H CH₂OH BrFor the following molecules, draw all possible stereoisomers using line-angle formulas with wedge/dash notation. Label the stereocenters as R or S. Indicate which ones are enantiomers and which ones are diastereomers. (Remember: there are 2 stereoisomers possible for a molecule with n chiral centers) (A) (B) (C) (1 chiral center) Br OH (2 chiral centers) CI OH CH3 (3 chiral centers)
- 10 Label the stereocenters present in the molecules below as either Ror S. (a) Cl (b) Cl (c) Cl (d) Cl (e) Cl (f)Draw all the possible stereoisomerism for following structures. Label the chiral centrewith an asterisk (*) if any.(a) CH(COOH)(NH2)CH2COOH(b) (CH3)2C=C(COOH)CH(NO2)CH3 PLEASE PROVIDE CLEAR DRAWING AND SOLUTIONSWhich of the following statements is/are true about the mirror-image stereoisomer of the molecule, shown below? OH OH HO i) The mirror-image stereoisomer of the compound shown has identical biological activity. ii) The mirror-image stereoisomer of the compound shown has the same melting point. iii) The mirror-image stereoisomer of the compound shown rotates plane-polarized light by the same amount but in the opposite direction. O only ii and iii are true O all of the statements are true O only i and iii are true O none of the statements are true O only i and ii are true
- 8. Two structures of Lipitor (a drug used to lower cholesterol) are shown below. (a) Determine the absolute configuration of each indicated stereocenter. Fill in the correct circle. (b) Determine if the two structures are the same compound or stereoisomers. Fill in the correct circle. (a) НО. Carbon a HO O OH Carbon a: OR OS Carbon b H N Carbon b: R OS of H Carbon c: OR OS OH OH Carbon c F Carbon d: R OS OH Carbon d (b) The two structures are: O the same compound O stereoisomersDraw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are percent in cyclopropane? What fraction of the overall 115 kJ/mol (27.5 kcal/mol) strain energy of cyclopropane is due to torsional strain.

