Given the following information on reduction potentials, calculate the standard free energy in kJ/mol based on your understanding of electron transfer through the pyruvate dehydrogenase complex. Round to nearest whole number. Lipoamide + 2H+ + 2e- dihydrolipoamide Ae° = -0.29 FAD + 2H+ + 2e- FADH, Aɛ° = -0.01
Given the following information on reduction potentials, calculate the standard free energy in kJ/mol based on your understanding of electron transfer through the pyruvate dehydrogenase complex. Round to nearest whole number. Lipoamide + 2H+ + 2e- dihydrolipoamide Ae° = -0.29 FAD + 2H+ + 2e- FADH, Aɛ° = -0.01
Chapter29: The Organic Chemistry Of Metabolic Pathways
Section29.SE: Something Extra
Problem 24MP: One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of xylulose...
Related questions
Question

Transcribed Image Text:Given the following information on reduction potentials, calculate the standard free energy in kJ/mol
based on your understanding of electron transfer through the pyruvate dehydrogenase complex.
Round to nearest whole number.
Lipoamide + 2H+ + 2e-
dihydrolipoamide Aɛ° = -0.29
FAD + 2H+ + 2e-
FADH, Aɛ° = -0.01
Expert Solution
Step 1
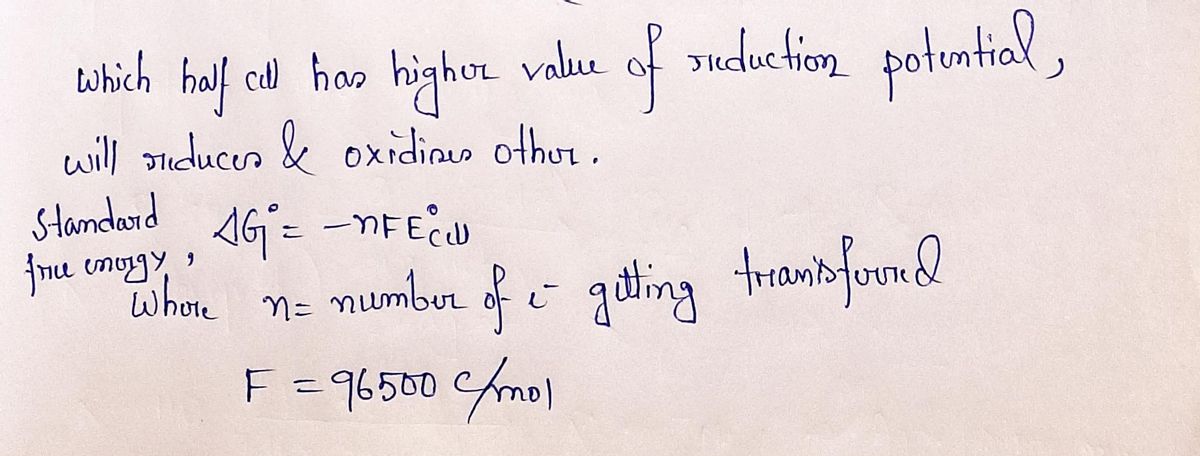
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning