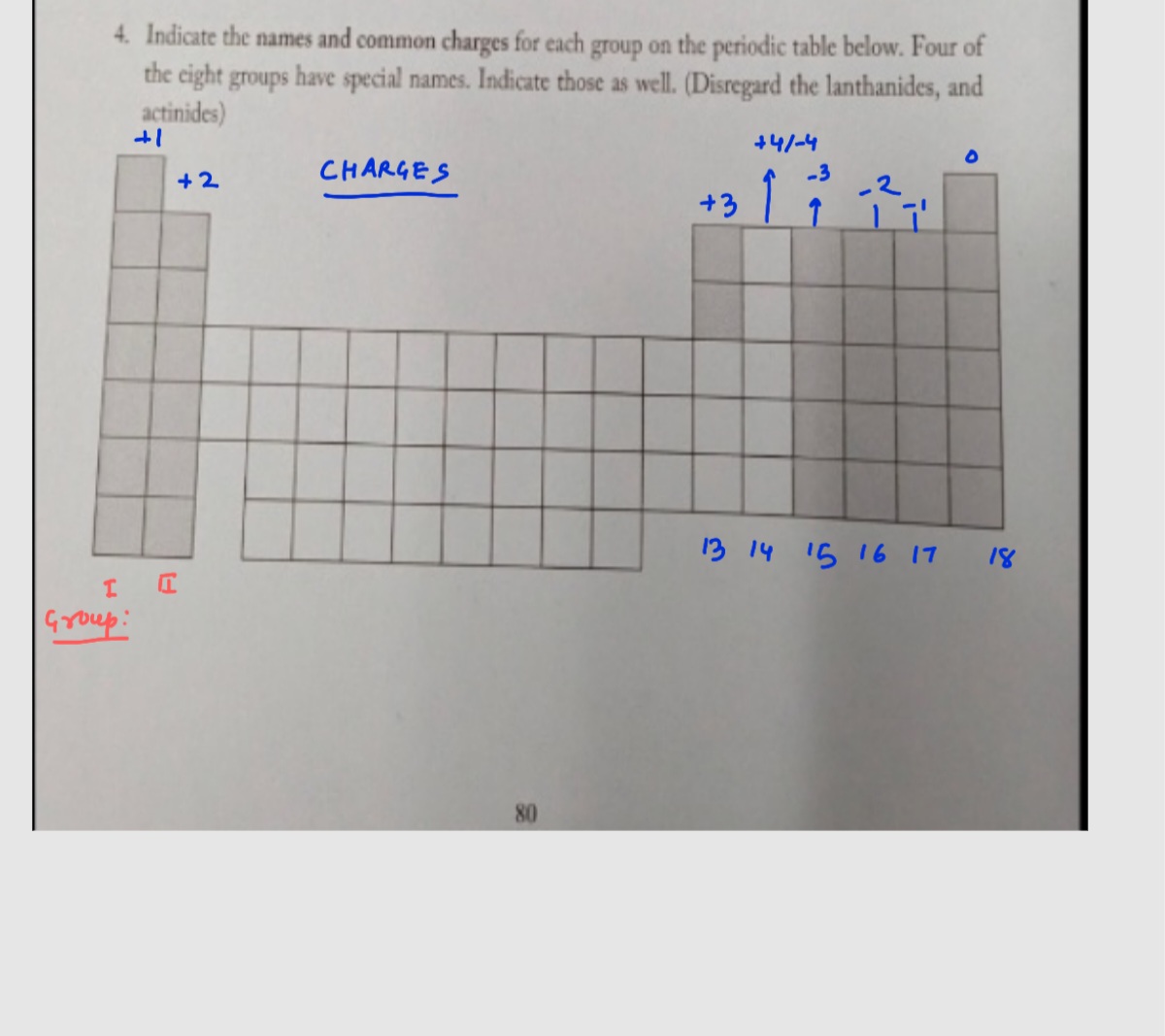
Fill in the empty Boxes with the correct information. Cation Anion Ca NH₂ CH CIO, Formula K₂S MgS Name Lithium Carbonate. 4. Indicate the names and common charges for each group on the periodic table below. Four of the eight groups have special names. Indicate those as well. (Disregard the lanthanides, and actinides)
Fill in the empty Boxes with the correct information. Cation Anion Ca NH₂ CH CIO, Formula K₂S MgS Name Lithium Carbonate. 4. Indicate the names and common charges for each group on the periodic table below. Four of the eight groups have special names. Indicate those as well. (Disregard the lanthanides, and actinides)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter22: The Chemistry Of The Transistion Elements
Section: Chapter Questions
Problem 44GQ: A platinum-containing compound, known as Magnuss green salt, has the formula [Pt(NH3)4][PtCl4] (in...
Related questions
Question
Already submitted but need help with number 4 also. Thank you.

Transcribed Image Text:3. Fill in the empty boxes with the correct information.
Cation
Ca
NH
Anion
CI
CIO,
Formula
K₂S
MgS
Name
Lithium Carbonate
4. Indicate the names and common charges for each group on the periodic table below. Four of
the eight groups have special names. Indicate those as well. (Disregard the lanthanides, and
actinides)
X
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
