Consider the following equilibrium between benzoic acid (Ka = 6.3x10) and its conjugate base: %3D H2O + H3O* (1) Will raising the pH shift the equilibrium to the right or to the left? (2) Calculate the ratio of the concentration of benzoate ion to the concentration of benzoic acid at a pH of 5.65. ratio [conjugate base] / [acid] =
Consider the following equilibrium between benzoic acid (Ka = 6.3x10) and its conjugate base: %3D H2O + H3O* (1) Will raising the pH shift the equilibrium to the right or to the left? (2) Calculate the ratio of the concentration of benzoate ion to the concentration of benzoic acid at a pH of 5.65. ratio [conjugate base] / [acid] =
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 94QRT:
Several acids and their respective equilibrium constants are:
Which is the strongest acid?
Which...
Related questions
Question
![[Review Topics]
[References]
Use the References to access important values if needed for this question.
Consider the following equilibrium between 3-methylbenzoic acid (K,a-5.0x10) and its conjugate base:
%3D
CH3-
CH3-
H2O
+ H30*
(1) Will lowering the pH shift the equilibrium to the right or to the left?
(2) Calculate the ratio of the concentration of 3-methylbenzoate ion to the concentration of 3-methylbenzoic acid at a pH of 1.59.
ratio [conjugate base] / [acid]
Submit Answer
Retry Entire Group
9 more group attempts remaining](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6cd0ba54-9c2f-4092-9f6d-bd36e9a12b87%2F002c6803-e4f5-4297-9f33-fe65def68b80%2Fw3s3dhd_processed.jpeg&w=3840&q=75)
Transcribed Image Text:[Review Topics]
[References]
Use the References to access important values if needed for this question.
Consider the following equilibrium between 3-methylbenzoic acid (K,a-5.0x10) and its conjugate base:
%3D
CH3-
CH3-
H2O
+ H30*
(1) Will lowering the pH shift the equilibrium to the right or to the left?
(2) Calculate the ratio of the concentration of 3-methylbenzoate ion to the concentration of 3-methylbenzoic acid at a pH of 1.59.
ratio [conjugate base] / [acid]
Submit Answer
Retry Entire Group
9 more group attempts remaining
![Consider the following equilibrium between benzoic acid (K, = 6.3×10°) and its conjugate base:
%3D
OH +
+ H3O*
-0.
(1) Will raising the pH shift the equilibrium to the right or to the left?
(2) Calculate the ratio of the concentration of benzoate ion to the concentration of benzoic acid at a pH of 5.65.
ratio [conjugate base] / [acid] =
Submit Answer
Retry Entire Group
9 more group attempts remaining](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6cd0ba54-9c2f-4092-9f6d-bd36e9a12b87%2F002c6803-e4f5-4297-9f33-fe65def68b80%2Fb1b2mb_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Consider the following equilibrium between benzoic acid (K, = 6.3×10°) and its conjugate base:
%3D
OH +
+ H3O*
-0.
(1) Will raising the pH shift the equilibrium to the right or to the left?
(2) Calculate the ratio of the concentration of benzoate ion to the concentration of benzoic acid at a pH of 5.65.
ratio [conjugate base] / [acid] =
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
Step 1
Since you have posted multiple questions, we are entitled to answer the first only.
The equilibrium reaction given is,
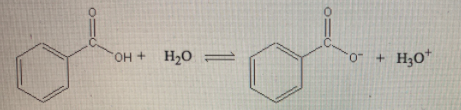
Given : Ka of reaction = 6.3 X 10-5
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning